chem exam questions
1/6
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
7 Terms
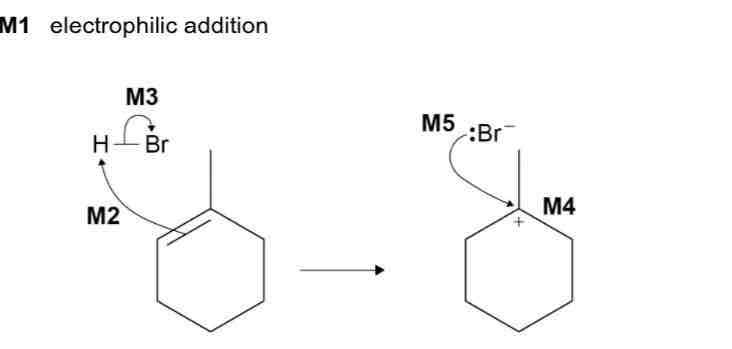
1-Methylcyclohexene reacts with HBr to form two structural isomers.
The major product is 1-bromo-1-methylcyclohexane.
Name and outline the mechanism for the formation of this major product.
alkene → halogenoalkane
Test for aldehyde
tollens reagent
silver mirror
state the meaning of the term fraction
mixture of compounds with similar boiling points
state what is meant by the term carbon neutral
no net emissions of carbon dioxide to the atmosphere
give one reason why carbon dioxide absorbs infrared radiation
C=O bonds are polar
state how figure 1 shows that the rate of reaction of 1-iodobutane is faster than the rate of reaction of 1-bromobutane
gradient of the line is steeper, because the C-I bond is weaker than the C-Br bond so breaks more easily