18.2-18.3: Stability of Nuclei and Energetics of Nuclear Reactions
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
11 Terms
When a nucleus forms, some of the mass of the separate nucleons is converted into energy (_________). The difference in mass between the separate nucleons and the combined nucleus is called the _________.
binding energy , mass defect
The greater the binding energy per nucleon, the ________ stable the nucleus is. Binding energy ________ as the nuclear mass _________.
more , increases , increases
State the equation used for calculating binding energy (E). How is it calculated?
∆E = (∆m)c2
Calculated by converting into energy units the mass difference (i.e. mass defect) between the sum of the masses of the free nucleons and the actual mass of the nucleus
The ∆Hrxn for nuclear reactions is called the __________. The equation for calculating it is __________ and then converting it to energy units (8.987 × 1010 kJ = 1 mol amu). A positive __________ indicates an __________ reaction while a negative __________ indicates an ___________.
Q-value , Q = ∑massesproducts - ∑massesreactants , Q-value , endergonic , Q-value , exergonic
The ratio of neutrons : protons (N/Z) is an important measure of _________ of the nucleus. If the N/Z ratio is too high, neutrons are converted to _________ via _________. If the N/Z ratio is too low, protons are converted to __________ via _________ or ________ (or α decay, but not as efficient).
stability , protons , ß decay , neutrons , positron emission , electron capture
Most stable nuclei have _________ numbers of protons and neutrons. Only a few don’t. If the __________ adds to a “magic number,” the nucleus is more stable.
even , total number of nucleons
The nucleus is most stable when N or Z = __________.
2, 8, 20, 28, 50, 82; or N = 126
What is the “band of stability”?
Area on a graph comprised of the stable nuclides of each element. Typically plotted with the number of neutrons on the y-axis and the number of protons on the x-axis; bordered by unstable neutron-rich and neutron-poor nuclides that undergo radioactive decay
List the stable N/Z value for each range of Z: 1-20, 20-40, 40-80, and Z > 83.
N/Z = 1, N/Z = 1.25, N/Z = 1.5, there are no stable nuclei
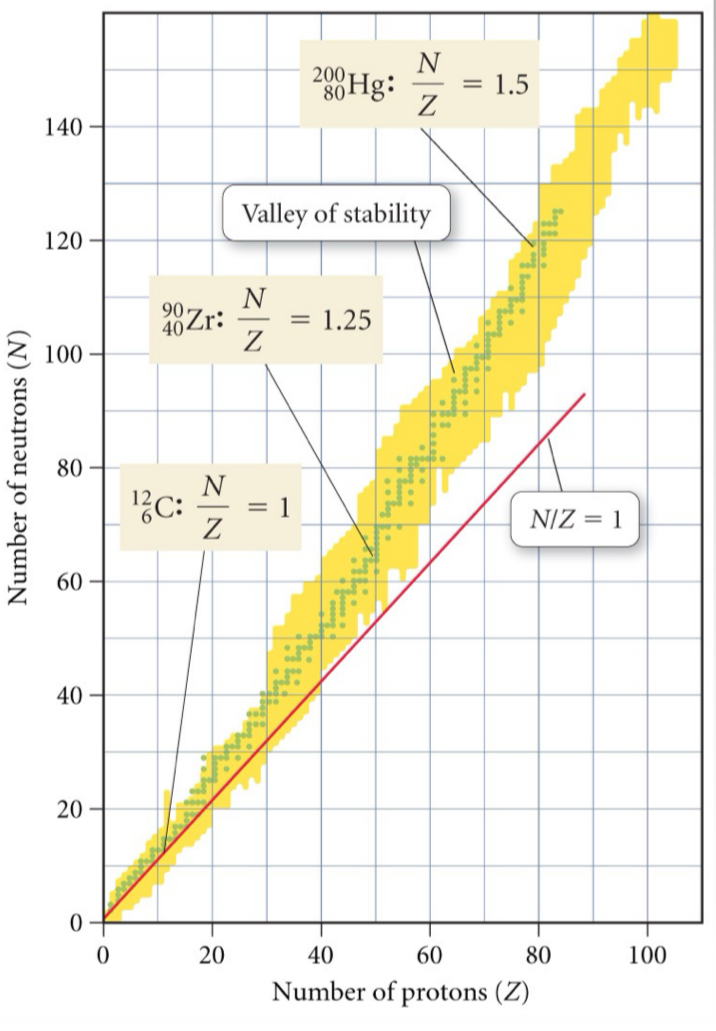
What are the steps for predicting radioactive decay?
Determine the Z and N values present for the given nuclide.
Divide N by Z and compare this value to the stable N/Z value for the element.
Could also compare the atomic mass (A) for the given isotope and the average atomic mass of that element in the periodic table
If the N/Z ratio is too high, neutrons will convert protons through Beta emission/decay.
If the N/Z ratio is too low, protons will convert to neutrons through positron emission or electron capture
What is a “decay series”? How do we determine the stable nuclide at the end?
All of the radioactive nuclides that are produced one after the other until a stable nuclides is made
Steps to determining the stable nuclide formed:
Count the number of α and ß decays
From the mass no. subtract 4 for each α decay
From the atomic no. subtract 2 for each α and ß decay and add 1 for each ß