Topic 1: Atomic Structure and the Periodic Table
1/35
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
36 Terms
The electrons
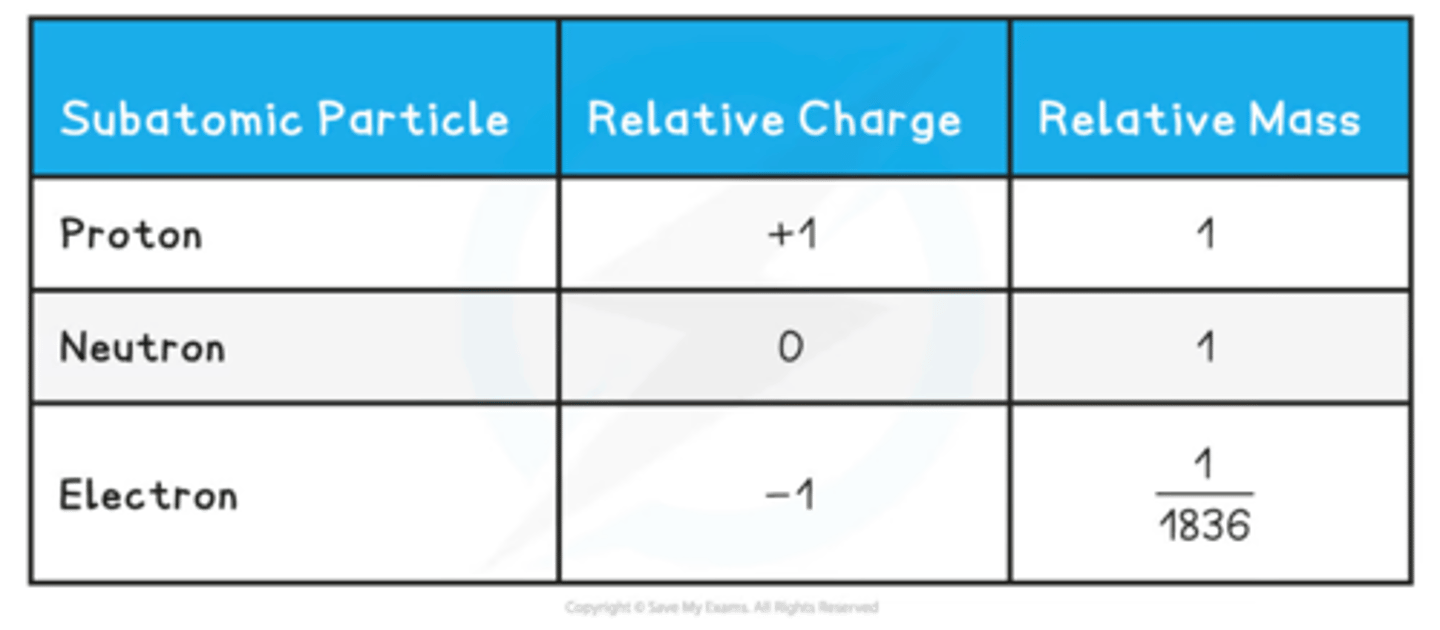
Move around the nucleus in electron shells. They're negatively charged and tiny. The volume of their orbits determines the size of the atom. They have virtually no mass.
Give the relative mass and relative charge of protons, neutrons and electrons
Why are atoms neutral?
The charge on electrons is the same size as the charge on protons, but opposite - so the charges cancel out. Whereas in an ion, the number of protons don't equal the number of electrons, so they have an overall charge.
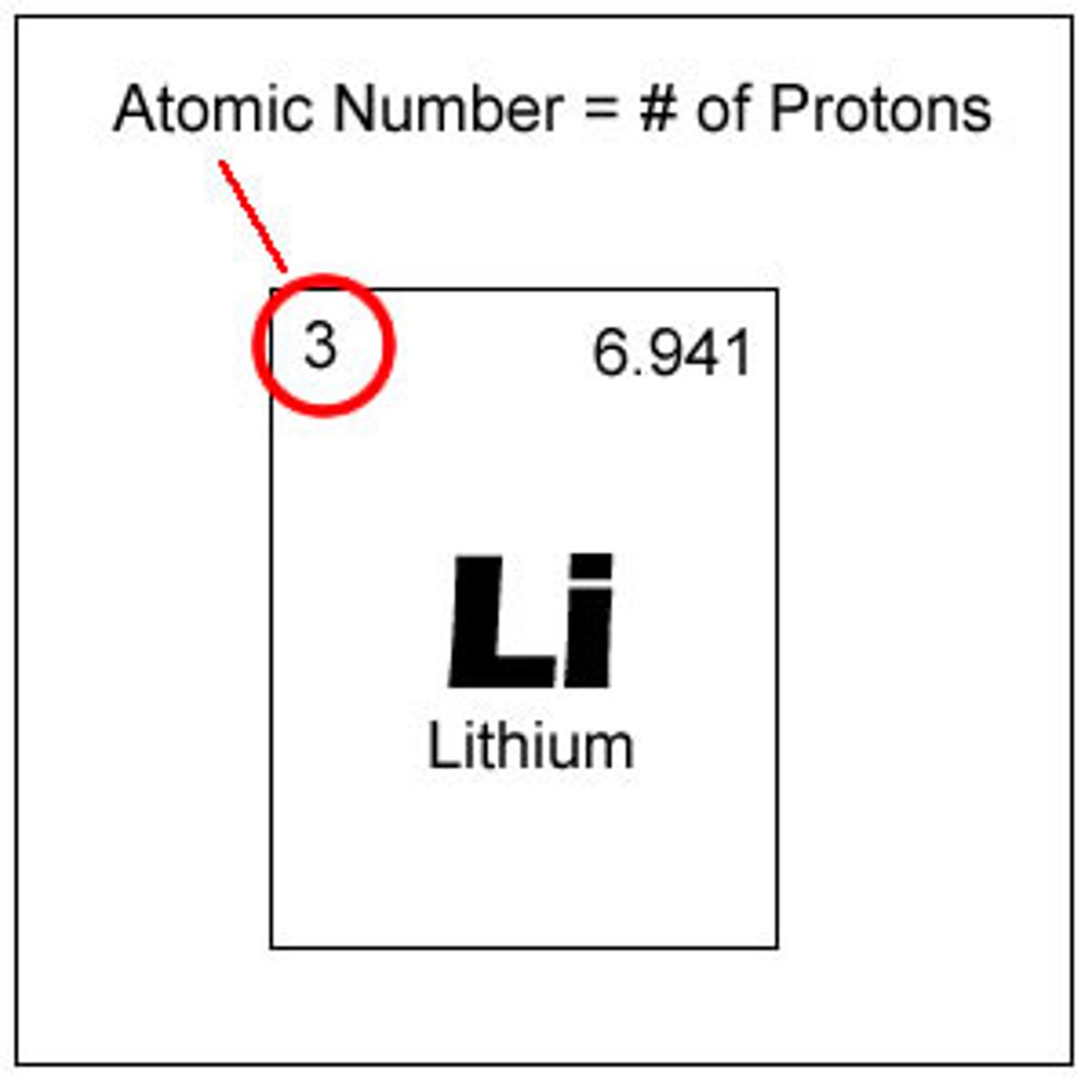
Atomic number
The number of protons in the nucleus of an atom.
What is the radius of an atom?
0.1 nanometres (1 x 10^-10 metres)
The nucleus
In the centre of the atom where most of the mass of the atom is concentrated, containing protons and neutrons. The protons give it a positive charge. The nucleus has a radius of 1 x 10^-14m.
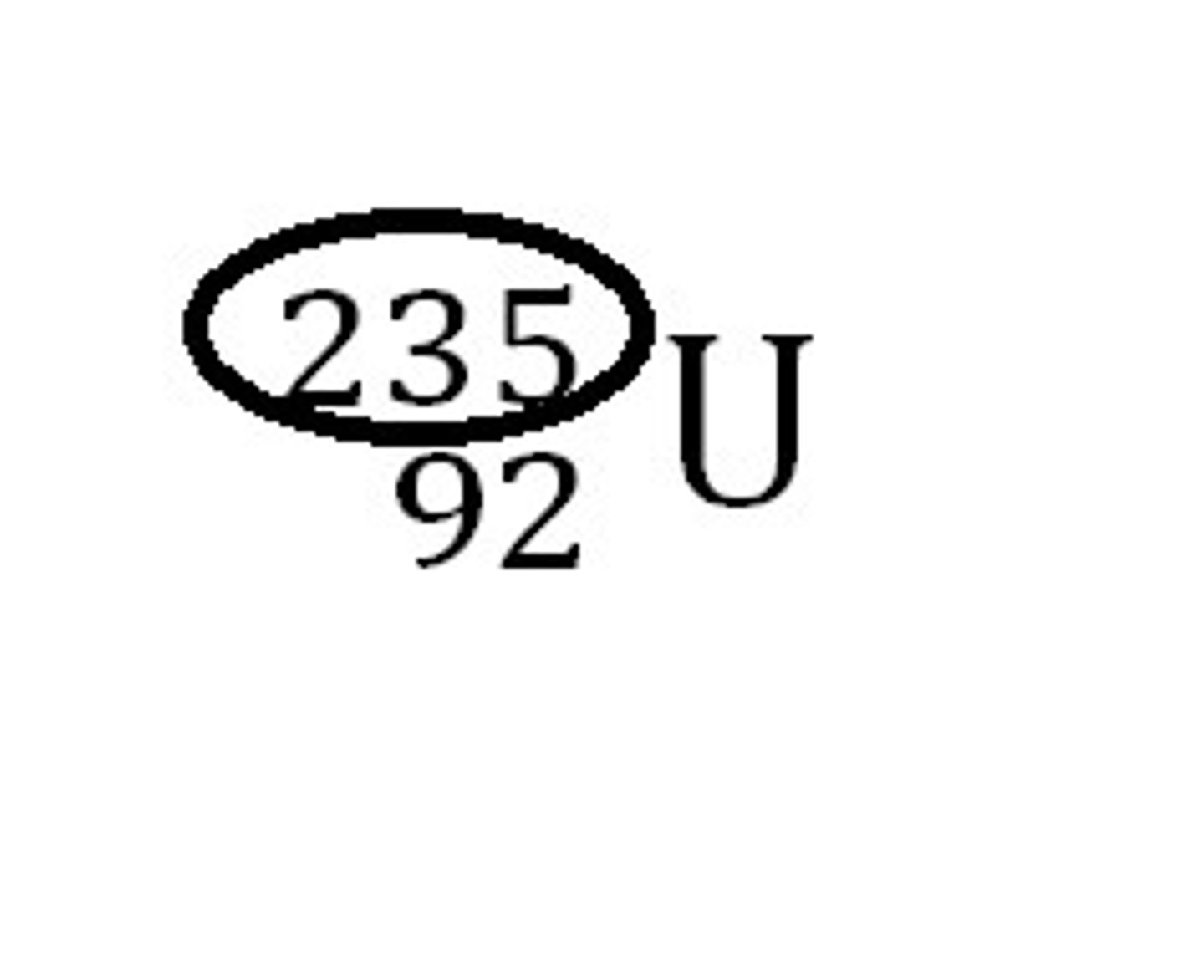
Mass number
The total number of protons and neutrons in the atom.
Elements
A molecule composed of one kind of atom (with the same atomic number); cannot be broken into simpler units by chemical reactions.
Isotopes
Atoms of the same element that have different numbers of neutrons (so different mass number).
Relative Atomic Mass
It is an average mass taking into account the different masses and abundances of all the isotopes that make up the element. Since many elements can exist as a number of different isotopes, relative atomic mass (Ar) is used instead of mass number when referring to the element as a whole.
Formula for calculating relative atomic mass
(isotope abundance x isotope mass number) + (isotope abundance x isotope mass number)/ sum of abundances of all isotopes (usually 100)
Compounds
2 or more elements chemically combined. A chemical reaction is needed to separate the original elements of a compound
Are properties of compounds the same or different from the elements they contain?
The properties of a compound are completely different from the properties of the original elements. For example, iron (a lustrous magnetic metal) and sulfur (a yellow powder) from the compound iron sulfide, which is a dull grey solid lump that behaves nothing like either element.
How are chemical bonds made?
It involves atoms giving away, taking or sharing electrons. Only electrons are involved, the nuclei of the atoms aren't affected at all.
Ionic bonding
A compound that is formed from a metal and a non-metal. The metal atoms lose electrons to form positive ions (cation) and the non-metal atoms gain electrons to form negative ions (anions). The opposite charges then mean that they're strongly attracted to each other.
Covalent bonding
A bond formed when non-metals share one or more pairs of electrons.
Mixtures
A combination of two or more substances that are not chemically combined. They can be separated out by physical methods.
Methods of separating mixtures
Filtration, simple or fractional distillation, crystallization and chromatography.
Are properties of mixtures the same or different from the elements they contain?
The properties are just a mixture of the separate elements, so they are not affected by it being part of a mixture.
REQUIRED PRACTICAL: Paper Chromatography
1) Draw a line near the bottom of a sheet of filter paper using a pencil as it won't dissolve in the solvent.
2) Add a spot of the ink to the line and place the sheet in a beaker of solvent, e.g. water.
3) Make sure the ink isn't touching the solvent as you don't want it to dissolve in it.
4) Place a lid on top of the container to stop the solvent evaporating.
5) The solvent will seep up the paper, carrying the ink with it, each different dye in the ink will move. Each different dye in the ink will move up the paper at a different rate so the dyes will separate out. Each dye will form a spot in a different place, 1 spot per dye in the ink.
6) If any dyes in the ink are insoluble in the solvent used they'll stay on the baseline.
7) When the solvent has nearly reached the top of the paper, take it out of the beaker and leave it to dry. The end result is a chromatogram.
Filtration
A process that separates insoluble solids from liquids.
What are the two methods of separating soluble solids from solutions?
1) Evaporation
2) Crystallisation
REQUIRED PRACTICAL: Evaporation
1) Pour the solution into an evaporating basin.
2) Slowly heat the solution to evaporate the solvent, making the solution get more concentrated and eventually crystals will start to form.
3) Keep heating the evaporating dish until all you have left are dry crystals.
REQUIRED PRACTICAL: Crystallisation
1) Pour the solution into an evaporating basin and gently heat the solution. Some of the solvent will evaporate making the solution more concentrated.
2) Once about half of the solvent has evaporated, remove the basin from the heat and leave the solution to cool.
3) The remaining salt should start to form crystals as it becomes insoluble in the cold, highly concentrated solution.
4) Filter the crystals out of the solution and leave them in a warm place to dry, like a drying oven.
REQUIRED PRACTICAL: Separating Rock Salt
1) Grinding - grind the mixture to make sure the salt crystals are small so will dissolve easily.
2) Dissolving - put the mixture in water and stir, the salt will dissolve but the sand won't. (Salt is soluble but sand isn't)
3) Filtering - filter the mixture, as the grains of sand won't fit through the tiny holes in the filter paper, while the salt passes through the filter paper as it's part of the solution.
4) Evaporation - evaporate the water from the salt to form dry crystals, or use crystallisation for larger crystals.
Simple distillation
Used to separate out solutions. E.g. to get pure water from seawater, as the water evaporates and is condensed and collected, until eventually you end up with just salt in the flask.
REQUIRED PRACTICAL: Simple Distillation
1) Heat the solution, and the part that has the lowest boiling point evaporates first.
2) The vapour is then cooled, condensed and collected.
3) The rest of the solution is left behind in the flask.
What is the problem with simple distillation?
It can only be used to separate things with very different boiling points. If the temperature goes higher than the substance with the higher boiling point, they will mix again.
Fractional distillation
Method to separate a liquid mixture into its individual components that have more similar boiling points.
REQUIRED PRACTICAL: Fractional Distillation
1) Put the mixture in a flask and put a fractionating column on top, then heat it.
2) The different liquids will all have different boiling points, so will evaporate at different temperatures.
3) The liquid with the lowest boiling point evaporates first. When the temperature on the thermometer matches the boiling point of this liquid, it will reach the top of the column.
4) Liquids with higher boiling points might also start to evaporate, however the column is cooler towards the top so they will only get part of the way before condensing and running back down the column.
5) When the first liquid has been collected, you raise the temperature until the next one reaches the top, and repeat until they have all been separated.
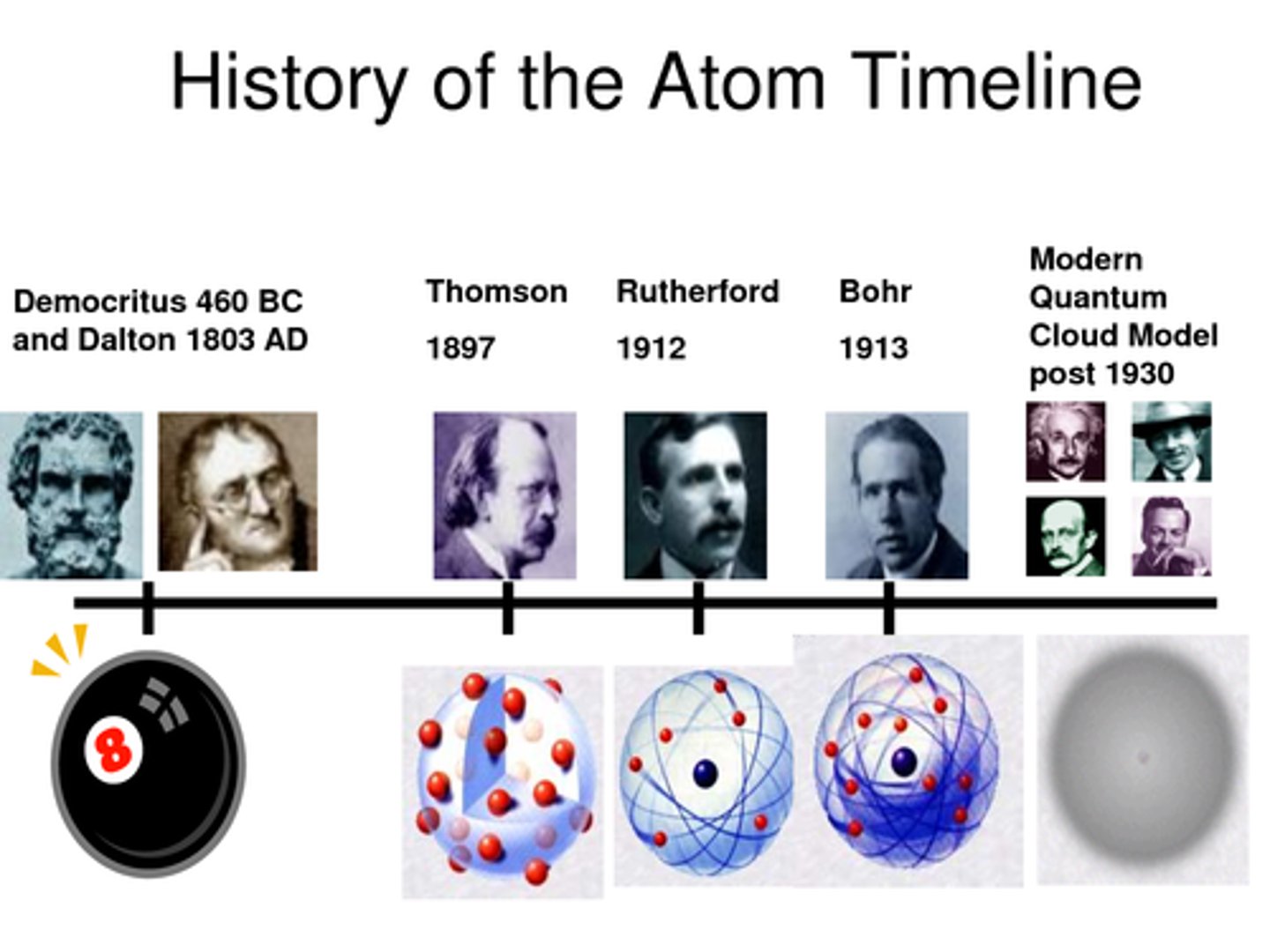
Who was involved in the History of the Atom?
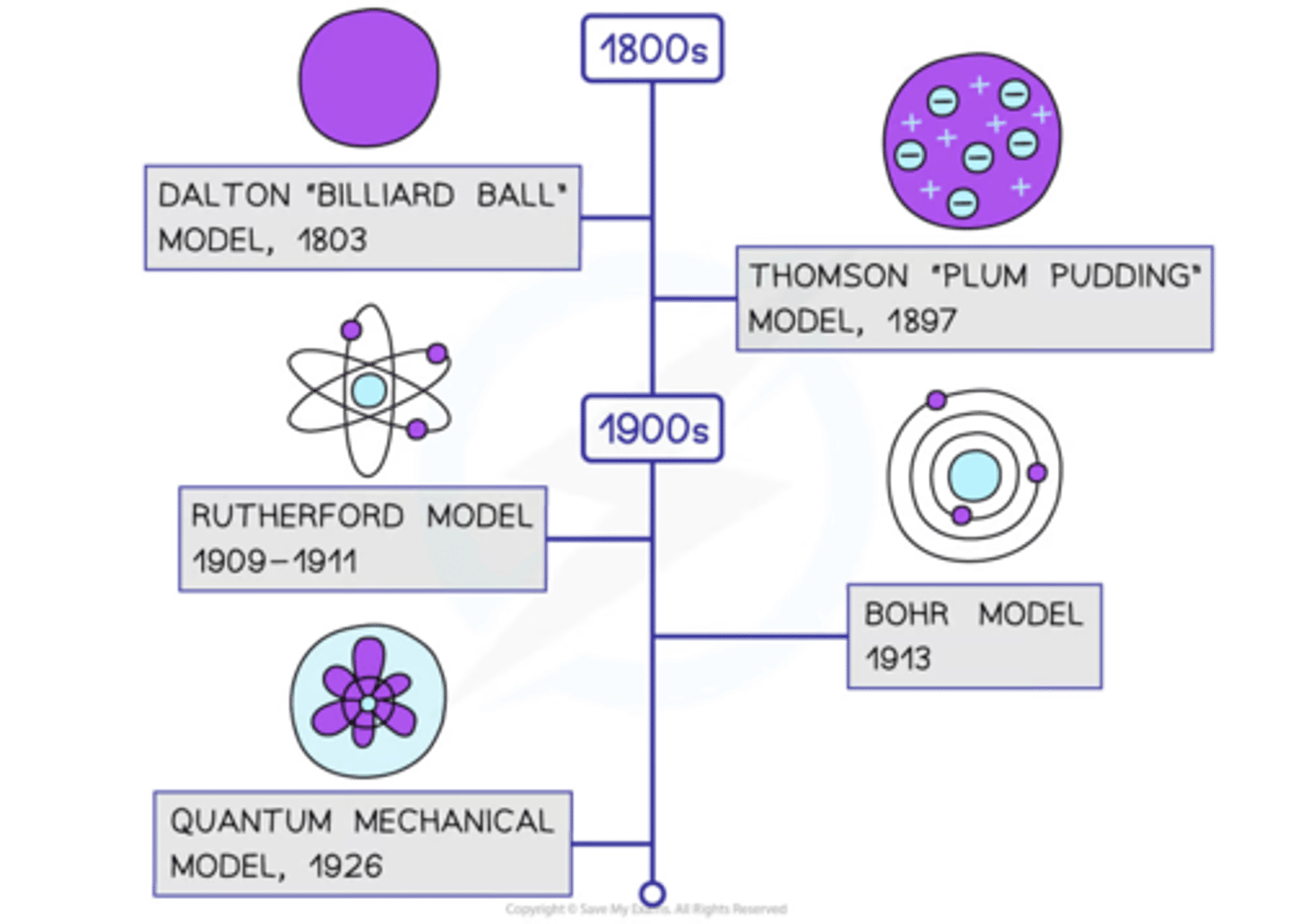
John Dalton
In 1803 presented his Atomic Theory: all matter is made of particles that can't be created, destroyed or divided; atoms of the same element are identical and vice versa; different atoms combine to form new substances.
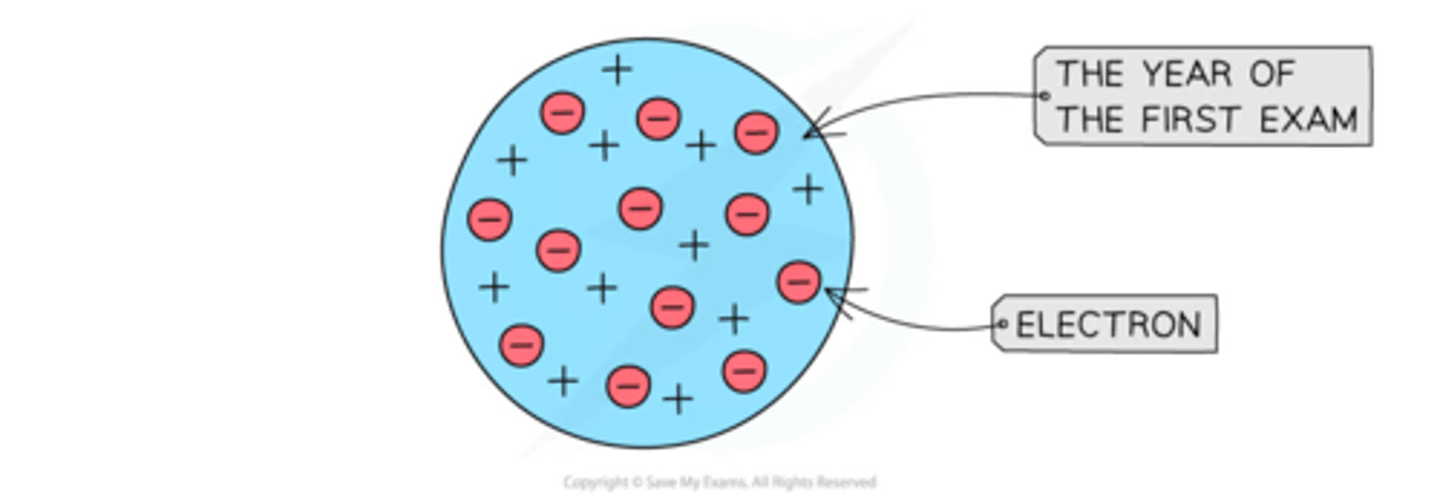
JJ Thomson's Plum Pudding Model
In 1897, Thomson discovered the electron. Using a cathode-ray tube, he conducted an experiment which identified the electron as a negatively charged subatomic particle. He used this to propose a model of the atom where negative electrons were embedded in a sphere of positive charge.
Ernest Rutherford's Nuclear Model
In 1909, Rutherford presented his model of the atom based on the famous gold foil experiment. Rutherford shot a beam of positively charged particles at a thin sheet of gold foil and found that although some particles were scattered, some were deflected back. This led to his discovery that most of an atom's mass is concentrated in the centre - called the nucleus - but was made up of mostly empty space.
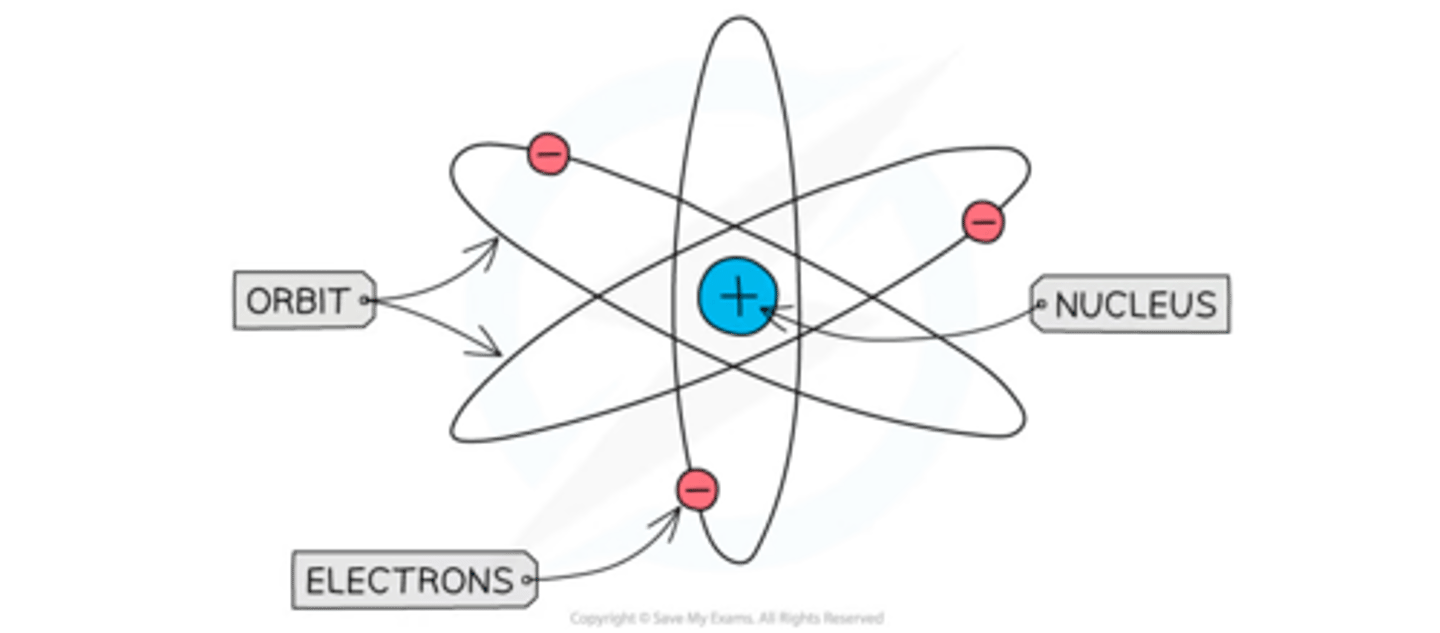
Niels Bohr
In 1913, Bohr proposed the idea that electrons orbit the nucleus in fixed shells. Further investigation revealed the nucleus could be divided into smaller particles, leading to the discovery of the proton.
How did the theory of atomic structure change throughout history
1) 1803 - John Dalton described atoms as solid spheres that make up the different elements
2) 1897 - JJ Thompson concluded atoms weren't solid spheres. An atom must contain smaller negatively charged particles (electrons). Called this the plum pudding model
3) 1909 - Ernest Rutherford conducted the gold foil experiment. Fired positively charged alpha particles at a thin sheet of gold, most particles went straight through. He concluded there was a positively charged nucleus at the center surrounded by a cloud of negatively charged electrons.
4) Niels Bohr made a new model where electrons orbited the nucleus in fixed shells.
5) Rutherford and others showed that the nucleus could be divided into smaller particles with the same charge as a hydrogen atom, these were known as protons.
6) James Chadwick provided evidence of the existence of neutral particles in the nucleus (neutrons).