Hydrolysis, Condensation, Triglycerides, Isomers, Reflux/Distillation
1/16
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Structural/Constituional Isomers
Compounds with the same molecular formula but different structural formula (atoms arranged in a different order)
eg move C atoms from parent to side chains, move functional group to dif C atom, draw a dif organic compound from the atoms
Geometric Isomers
Only present in Alkenes due to the C=C double bond which is rigid and the position of the atoms around it are fixed in place as the double bond cannot be rotated
Cis isomers = have same groups of atoms on same side as double bond
Trans isomers = have same groups of atoms on different sides of double
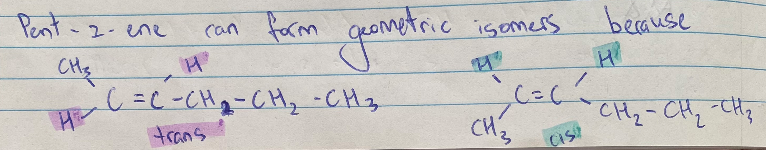
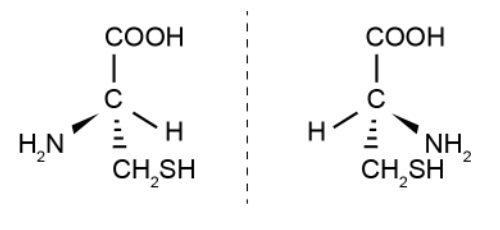
Optical Isomers
Compounds that have an asymmetrical chiral carbon, which is a carbon that is bonded to 4 different atoms or groups of atoms, can exist as enantiomers.
Enantiomers/optical isomers are mirror images of each other and non-superimposable. To differentiate them, shine plane polarised light through both solutions, one enantiomer will rotate the light in a clockwise direction and the other in an anti-clockwise direction but by the same amount
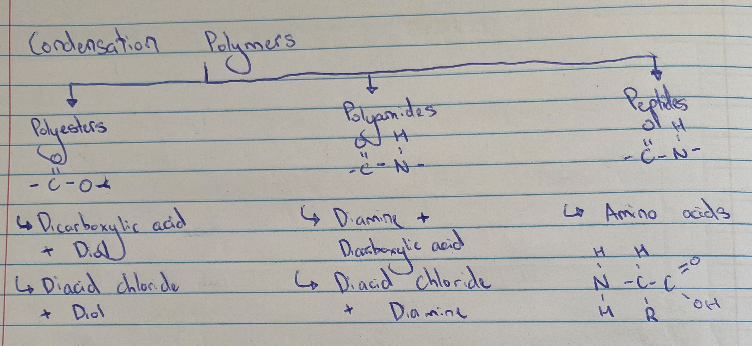
Monomers of Condensation Polymers
Instead of double bonds they have function groups (eg alc, amine, acyl chloride, carboxylic acid)
Each monomer has min 2 reactive sites, usually meaning 2 functional groups
The formation of POLYMER is an example of a condensation reaction. Hence, POLYMER is a condensation polymer. During the reaction MONOMER 1 will lose … while MONOMER 2 will lose … to eliminate H2O/HCl. The monomer molecules join using ESTER/AMIDE link to form a long chain molecule.
Acidic Hydrolysis
DILUTE strong acids eg HCl, dilute H2SO4 (H2O/H+) used
If any monomer contains a basic functional group, this will react with acidic solution in an acid-base reaction (transfer of H+ protons)
Basic Hydrolysis
DILUTE solution of strong base eg NaOH (aq), KOH (aq) used (OH- aq)
If any monomer contains an acidic functional group, this will react with the basic solution in an acid-base reaction (transfer of H+ protons)
Triglyceride Formation
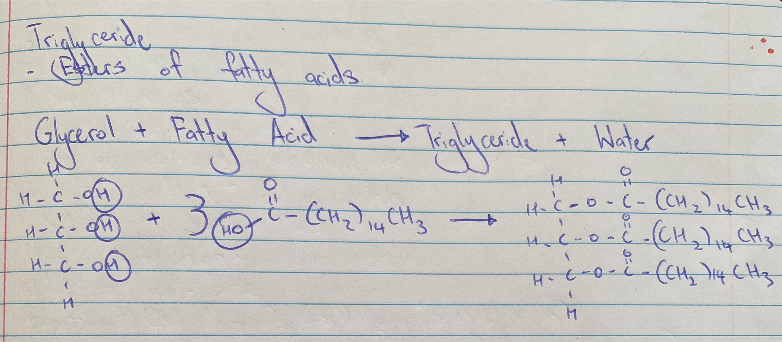
Condensation Polymers
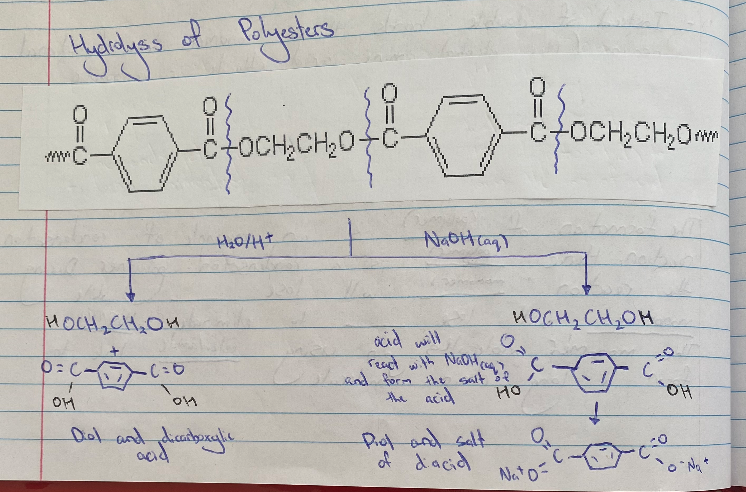
Polyester Hydrolysis
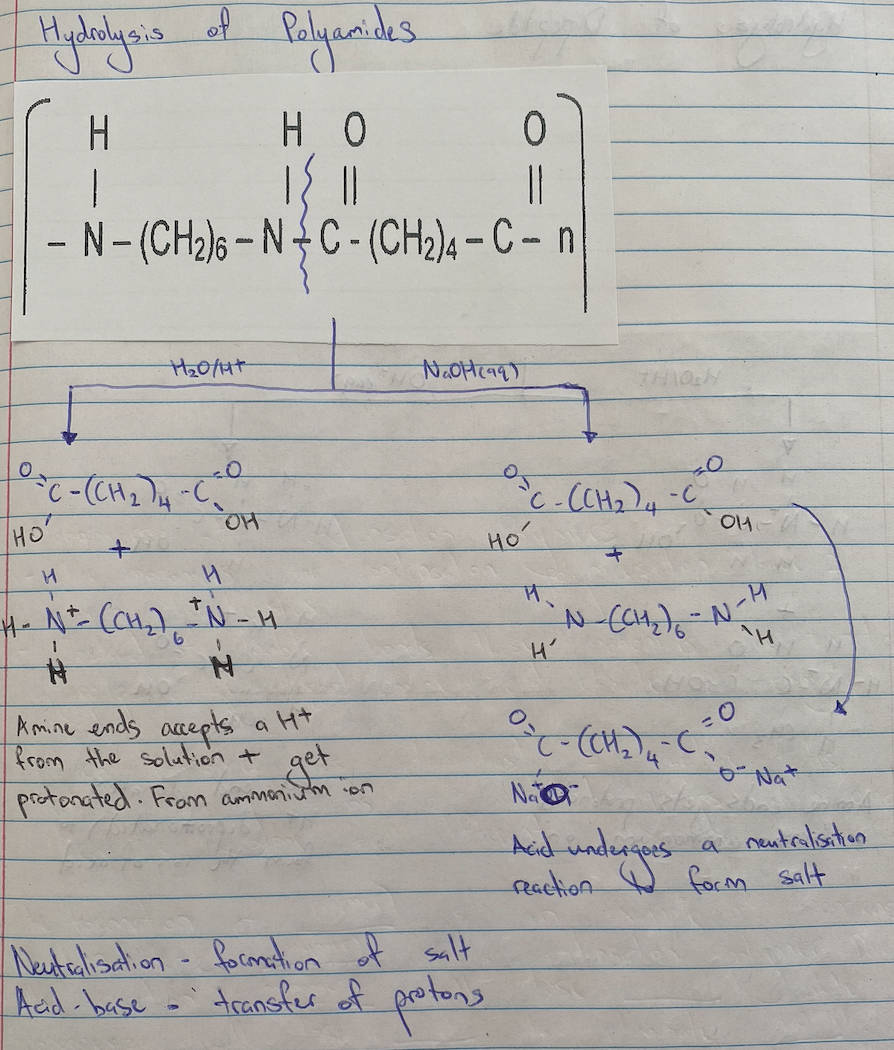
Polyamide Hydrolysis
Peptide Hydrolysis
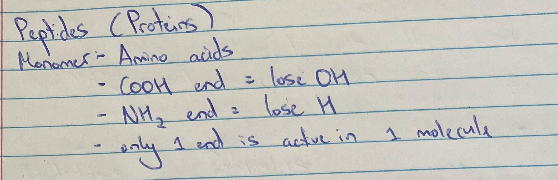
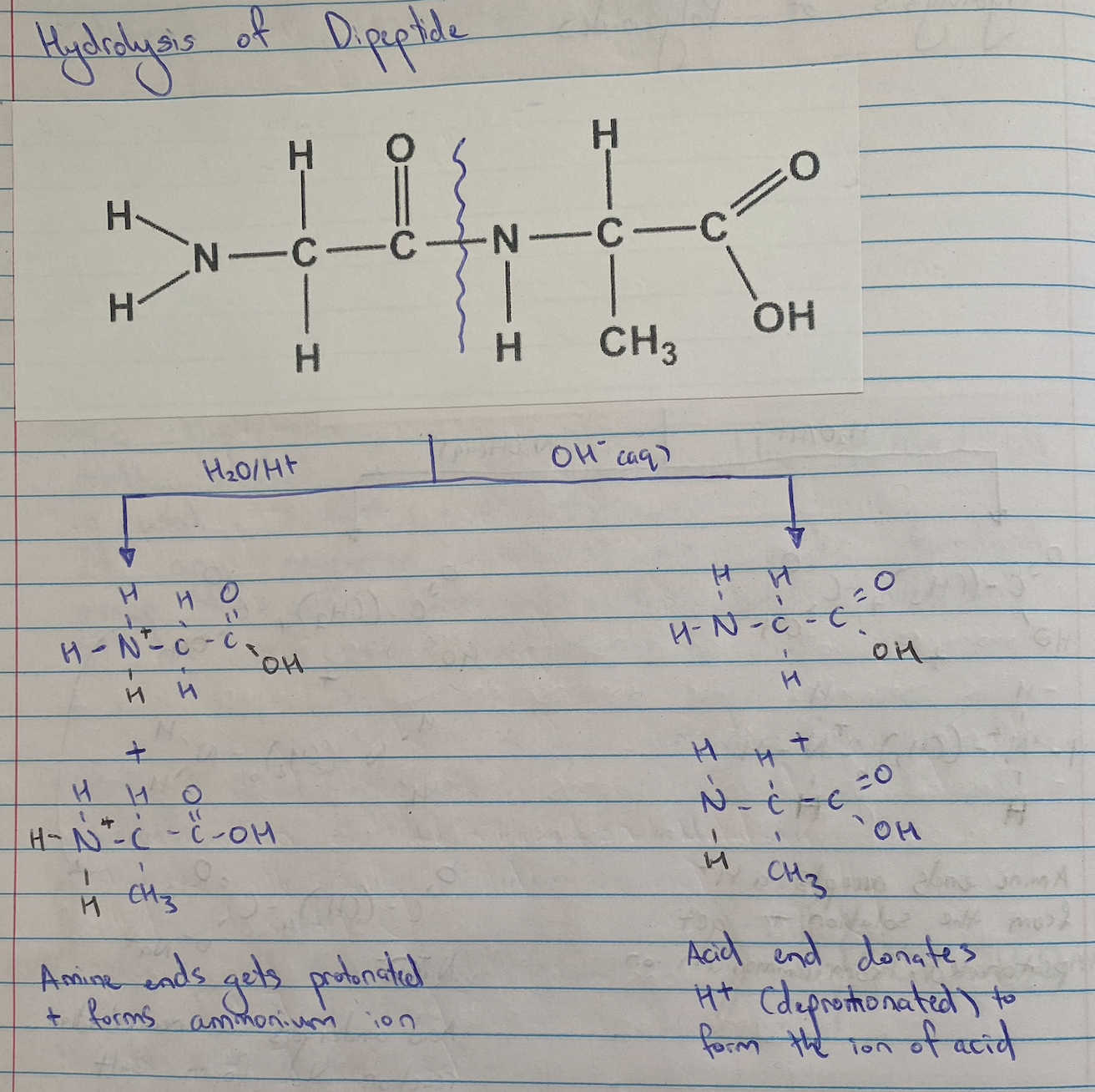
Dipeptide Hydrolysis
Hydrolysis of Amide
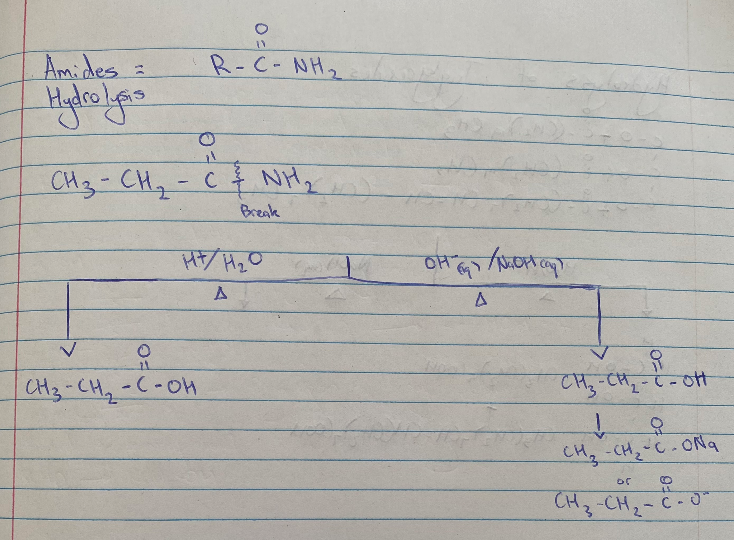
Triglyceride Hydrolysis

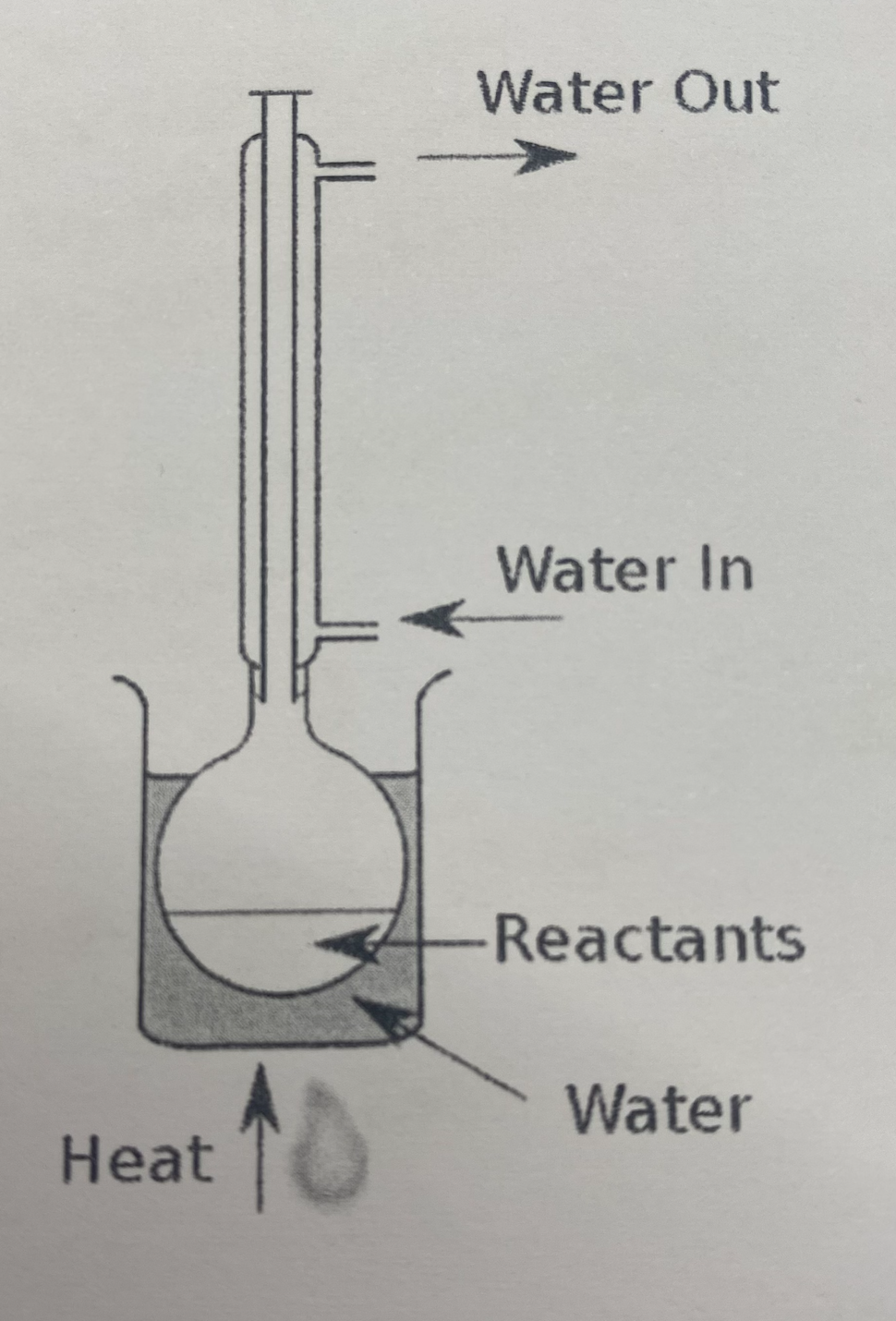
Reflux
When chemicals are heated together, the reaction mixture boils and the product and some reactants form gases which move up the condenser. The gases then liquefy when they reach the colder parts of the condenser and flow back down into flask.
Improves the yield/percentage of product produced
Max product formation since any unreacted reagent that vapourises returns to the flask
Speeds up the rate of reaction
Unreacted acid removed by adding Sodium Carbonate chips
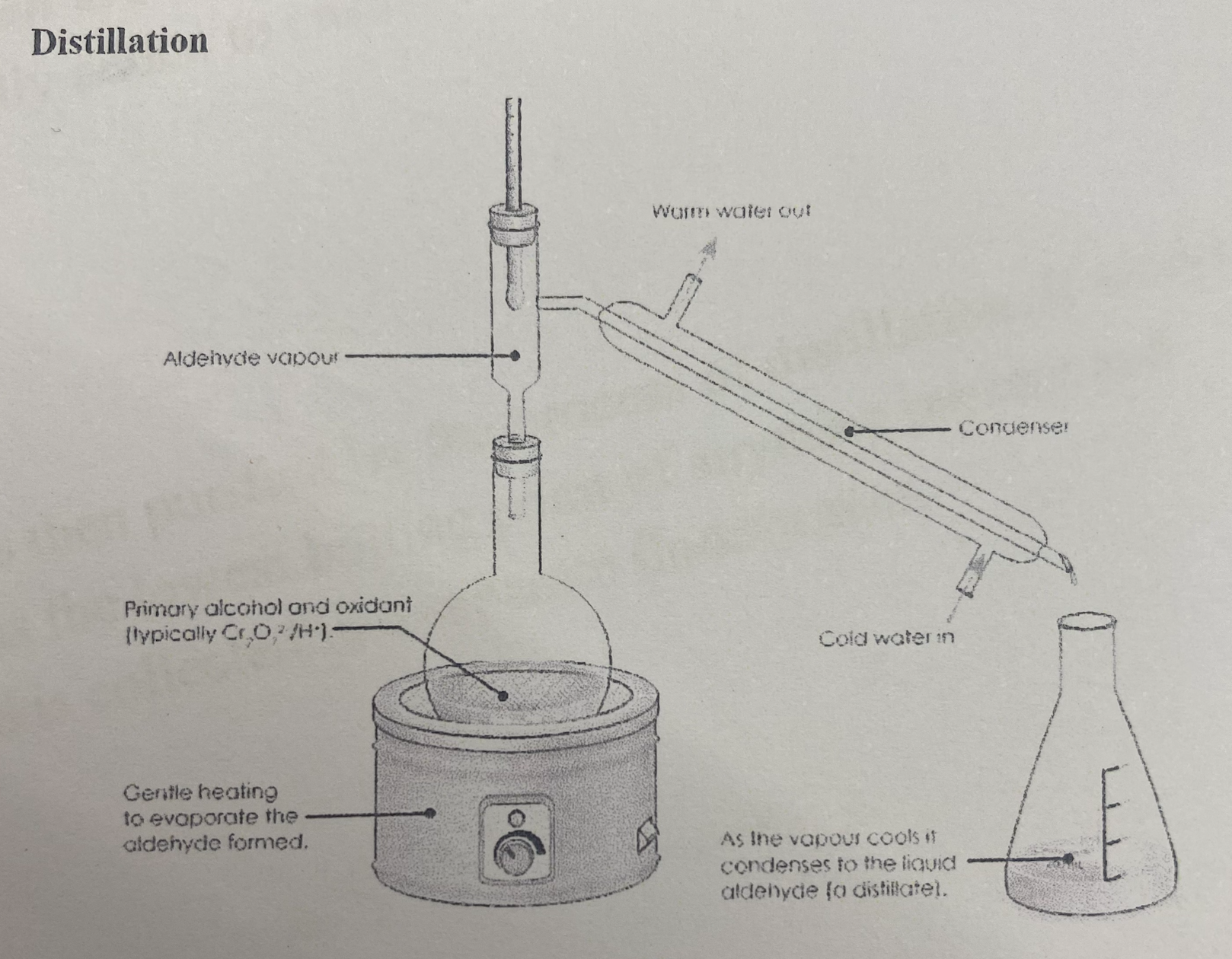
Distillation
When reactant and oxidant are heated, the product forms, which easily vaporise due to having a lower boiling point than the reactant. The vapour enters the condenser, attached horizontally at an angle to the reactant flask and filled with cold water. This condenses the product gas which flows down into a different flask where it can no longer take part in the reaction. Thus, the volatile product is removed from the reaction flask and is no longer oxidised. ,
Works on basis that each liquid has a dif boiling point
Used for partial oxidation of primary alc to aldehyde
Used to obtain PURE samples of the products
Condensation and Hydrolysis Definitions
Hydrolysis is a reaction where water is used to break down a large molecule into smaller molecules. The term "hydrolysis" literally means "water splitting" (hydro = water, lysis = to break).
Hydrolysis is the reverse of a condensation reaction, where water is removed to join molecules together.