Reactions of acids and metal carbonates
1/5
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Apparatus
25cm3 measuring cylinder
10cm3 measuring cylinder
Boiling tube
Test-tube
Disposable pipette/dropper
Test-tube rack
Hydrochloric acid
Calcium carbonate
Limewater
Method
Measure 15cm3 hydrochloric acid into the boiling tube using measuring cylinder
Measure 3cm3 limewater using small measuring cylinder into test tube
Keep tubes side by side in rack
Add calcium carbonate to acid in boiling tube
Using pipette, collect gas produced by opening and closing above reaction
Once collected bubble through limewater and record observations
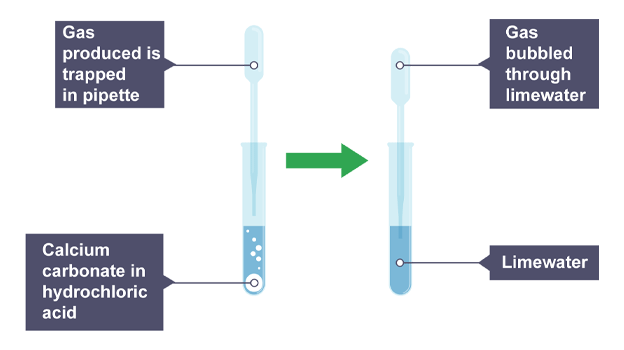
Observations
Gas produced
Fizzing
White solid disappears
Colourless solution formed
Limewater turns from colourless to milky (carbon dioxide)
Equation
acid + metal carbonate → salt + water + carbon dioxide
Error
collect gas efficiently/ quickly using pipette to avoid leakage
Safety
safety goggles to protect eyes from splashes
handle in well ventilated area/ fume cupboard
avoid skin contact with hydrochloric acid using gloves