Particle Model of Matter
1/34
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
35 Terms
matter
is anything that takes up space and has mass
law of conservation of mass
the idea that matter cannot be created or destroyed
physical properties
a property that can be measured or observed without changing the idenity of matter
solubility
the amount of a substance that can dissolve in a given amount of another substance
dissolve
given amount of another substance
physical change
when a charge occurs without a change in a substance's chemical composition at a molecular scale
phase change
when a substance goes from one state of matter to another, the process is a change in state
chemical composition
during a change in state, the substance changes its physical properties without changing its chemical composition
chemical properties
only observed when one attempts to change the identity of a substance
chemical reaction
a change (or lack of a change) in a substance must be observed during or after a chemical reaction
extensive property
one that depends directly on the amount of a substance present
intensive property
one that does not depend on the amount of the substance present
volume
extensive property, change with the amount of substance
mass
extensive property, change with the amount of substance
mixture
a blend of two or more kinds of matter, each of which retains its own identity and properties
pure substances
a fixed composition
homogeneous mixture
mixtures that have the same composition, or are uniform
heterogeneous mixture
mixtures that are not uniform throughout
compound
substances with constant composition that can be broken down into elements by chemical process
decomposed
substances with constant composition that can be broken down into elements by chemical processes
elements
cannot be broken down into simpler substances by chemical or physical means
solutions
mixtures that have the same composition, or are uniform, are said to be homogeneous
filtration
can separate components that have small differences in particle size
evaporation
mixtures can often be separated by using techniques such as filtration, evaporation, distillation, decanting, or centrifugation
distillation
separates components based on differences in the volatility of the components
homogeneous
mixtures that have the same composition, or are uniform
chemistry
is the study of the composition of matter, the physical and chemical changes that matter undergoes, and the energy absorbed or released during those changes
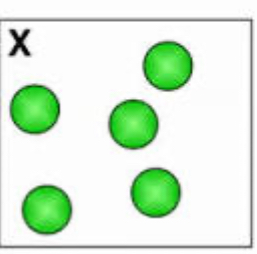
element, compound, or mixture?
element
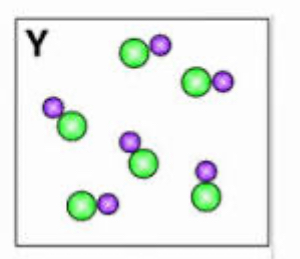
element, compound, or mixture?
compound
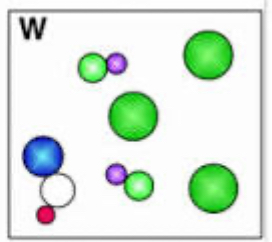
element, compound, or mixture?
mixture
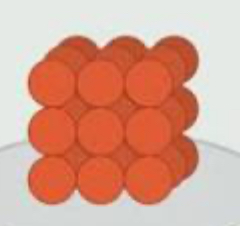
solid, liquid, or gas?
solid
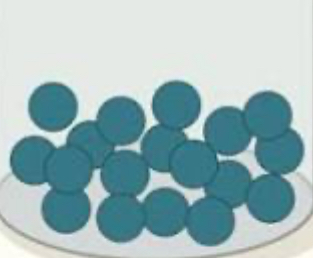
solid, liquid, or gas?
liquid
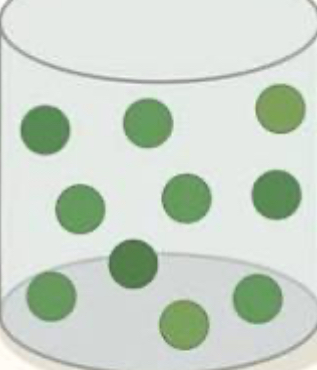
solid, liquid, or gas?
gas
Atoms
are the building blocks of matter
Atom
the smallest unit of an element that maintains
the identity of the element.