Reduction of Cyclohexene Oxide to Cyclohexanol (Lab 5)
1/10
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
11 Terms
In the reduction of cyclohexene oxide to cyclohexanol, the reducing agent is
LiBH4 which serves as a source of hydride
Cyclohexanol is extracted from the product mixture using
Methylene Chloride
The organic solution of cyclohexanol product is washed with
Water
If a student began the cyclohexanol cycle with exactly 10 g of cyclohexanol and obtained yield of 1.5 g of cyclohexanol product from the reduction of cyclohexene oxide, the students percent recovery of cyclohexanol is
15%
If a student starts with 1 mmol of cyclohexene oxide and obtains 0.5 mmol of cyclohexanol product after the reduction reaction, the students percent yield for the reduction reaction is
50%
lab 5 reaction
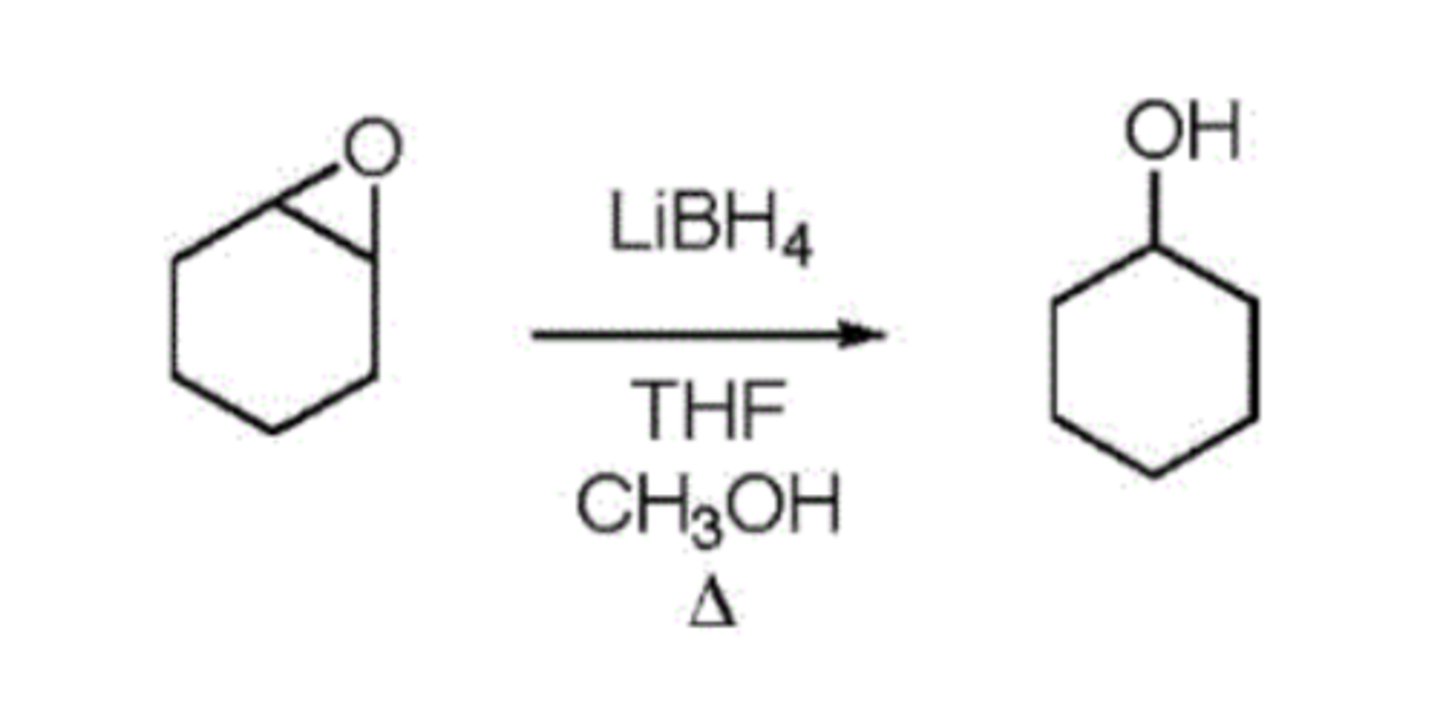
LiBH4 more soluble than
NaBH4 and LAH
solvent system used
THF and methanol
how many hours refluxed?
1.5-2
1:1 methanol water solution used to __
quench reaction using equal volume of THF started with
aqueous solution extracted with
two separate portions of DCM (Ch2Cl2)