Chemistry - History, Important Figures, and Trends of Atomic Theory + Figures of the Periodic Table
1/9
Earn XP
Description and Tags
Contains key dates, scientists, and models of the atom and the history and the Periodic Table.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
10 Terms

Democritus
Indivisible Particle
“Atomos“
Aristotle
All matter is
earth
fire
wind
water
Dmitri Mendeleev
“Father of the Modern Periodic Table“
organized by atomic weight
discovered periodic trends
predicted future elements
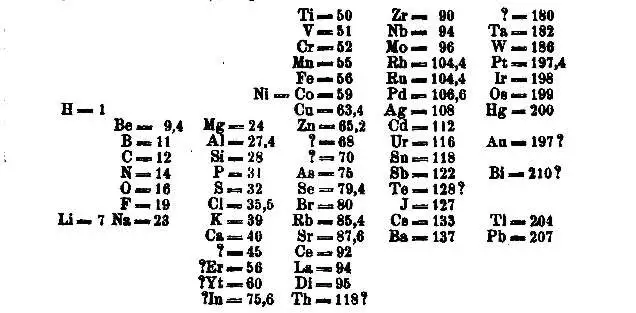
Henry Moseley
Reorganized periodic table
based on atomic number
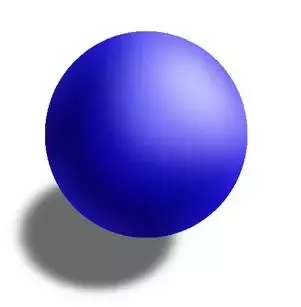
John Dalton (1808)
Published First Atomic Theory
All atoms of an element are identical
Solid Ball
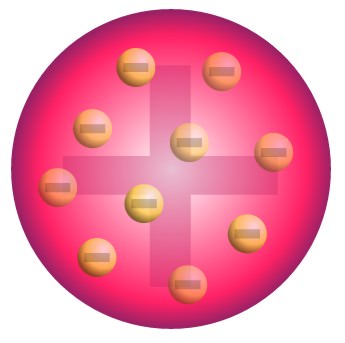
J.J. Thomson (1897)
Discovered electrons
Negative Particles in a Positive Atom
Plum Pudding Model
Ernest Rutherford (1911)
Atom is mostly empty space
Discovers positive nucleus
Performed Gold Foil Experiment

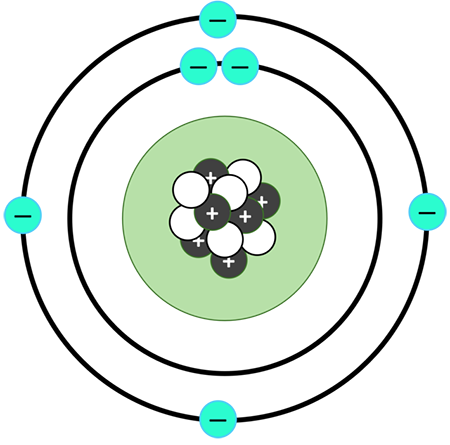
Niels Bohr (1913)
Electrons orbit the nucleus
Electron jumping
Planetary Model
Erwin Schrodinger (1926)
Electron Orbitals
Electron Cloud Model
James Chadwick (1932)
Discovered neutrons
Neutron Model