2.2 electrons and orbitals
1/78
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
79 Terms

What happens when short wavelength (high energy) light shines on a metal surface?
Electrons are emitted, creating a measurable current.
What happens when the intensity (brightness) of light is increased on the metal surface?
More electrons are emitted because more energy is shining on the metal.
Is there a frequency at which electrons stop being ejected from the metal surface?
Yes, there is a threshold frequency below which no electrons are ejected, regardless of the light's intensity.
What would happen if light were a wave in terms of intensity and energy?
Increasing the intensity would increase the energy and should eject electrons, which contradicts the photoelectric effect.
Photoelectric Effect
Only light above a certain threshold frequency (energy) will result in ejected electrons.
How did Einstein explain the photoelectric effect?
Einstein postulated that light comes in packets (particles or quanta) called photons.
How is energy transferred in the photoelectric effect?
Energy is transferred as a particle (photon) that has a definable energy.
What is the formula for the energy of a photon?
The energy of a photon is given by E=hν, where h (6.626e-34 J.s) is Planck's constant and
ν is the frequency of the light.
How many photons are required to eject one electron in the photoelectric effect?
One photon ejects one electron.
What happens if a photon doesn't have enough energy to eject an electron?
If the photon doesn't have enough energy, no electron is ejected.

B
What do spectra show about light from different elements?
Spectra show light only of specific wavelengths/energies, indicating the unique emission or absorption patterns of elements.
Does the spectrum of an element change depending on where it is located (e.g., Earth, Sun, or distant galaxy)?
No, the spectrum of an element is the same regardless of whether the element is on Earth, in the Sun, or in a galaxy light years away.
How did Rutherford's model describe the arrangement of electrons in an atom?
Electrons were thought to be circling the nucleus like planets around the Sun.
What problem did Rutherford's model present?
According to the model, the atom would have eventually "blown up" because charged electrons circling in an electric field would lose energy and decay.
Was Rutherford's model sustainable?
No, the model was not sustainable due to the issue of electron energy decay.
Who developed the next model of the atom after Rutherford's?
The next model was developed by Niels Bohr.
How did Niels Bohr describe the movement of electrons in an atom?
Electrons move in orbits around the nucleus.
What are the characteristics of these orbits in Bohr's model?
The orbits have definite energies and are at definite distances from the nucleus.
What does it mean for the energies of electrons in atoms to be quantized?
The energies of electrons in atoms are restricted to specific, discrete values.
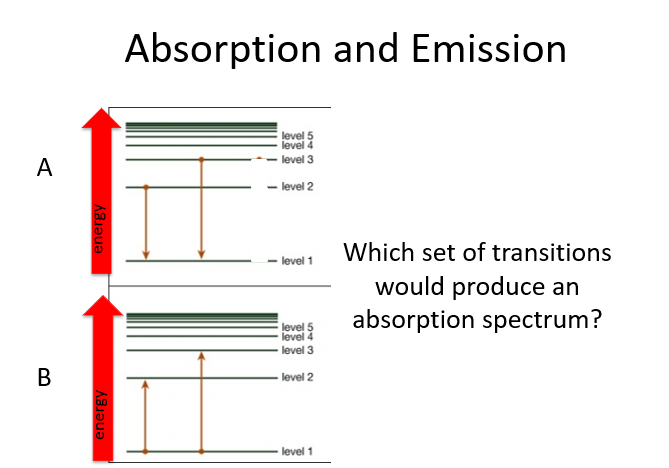
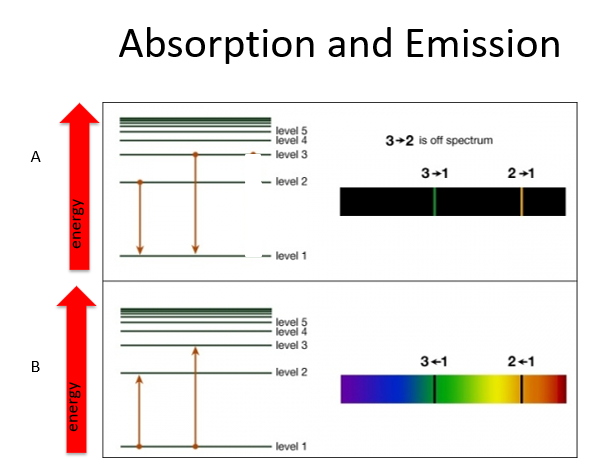
How did Bohr explain emission and absorption spectra?
He explained them by invoking discrete energy levels, characterized by quantum numbers (n).
What happens when electrons move between energy levels in Bohr's model?
Photons of electromagnetic energy are emitted or absorbed as electrons move from one energy level to another.
What determines the energy of the photons emitted or absorbed by an atom?
The energy of the photons corresponds to the difference in energy levels of the electrons.
What does each energy level in an atom have?
Each energy level has a quantum number.
How is energy related to the quantum number of an energy level?
The higher the quantum number, the higher the energy of the level.
Are energy levels the same as orbits?
No, energy levels are not orbits.
How do electrons transition between energy levels?
Electrons transition between energy levels by absorbing or emitting photons.
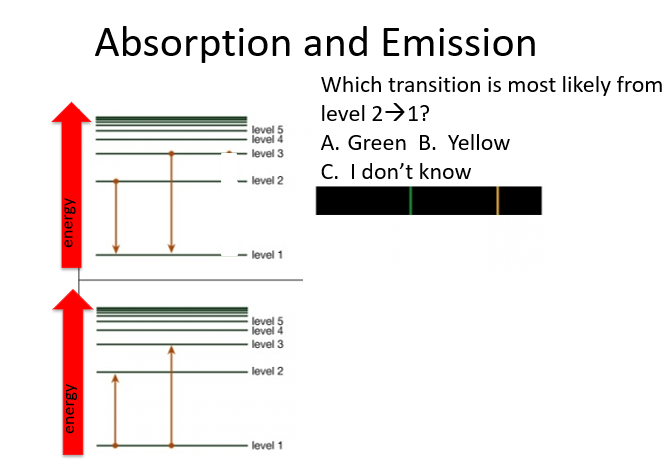
B
B
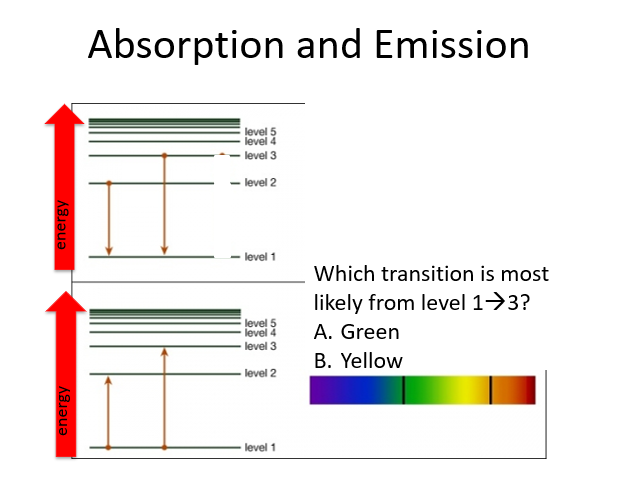
A
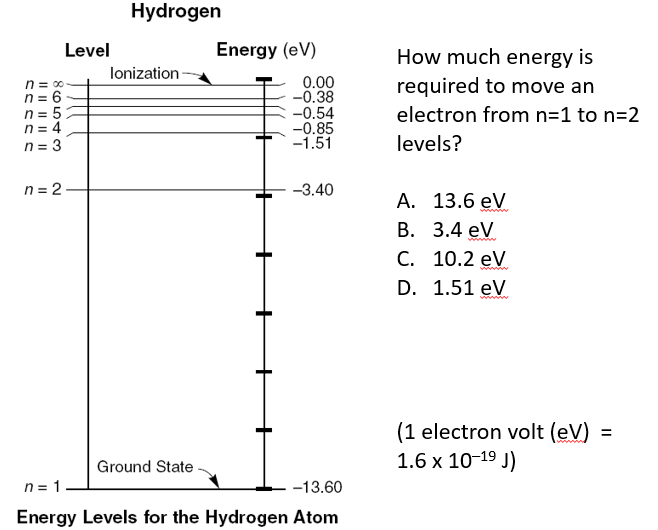
C
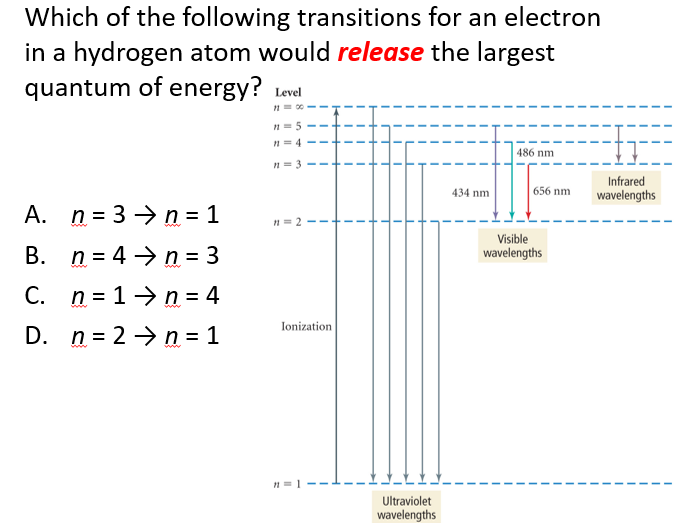
A
Why does Bohr’s model only work for hydrogen?
Bohr’s model only works for hydrogen because it has one electron, and the model can’t explain atoms with more than one electron.
What does the Heisenberg Uncertainty Principle state?
It states that you can't measure both the position and momentum (or energy or velocity) of a small particle, like an electron, with complete accuracy.
Why can't we use Bohr's model to describe electrons in atoms?
Bohr’s model specifies both the position (of the orbit) and energy of the electron, which contradicts the Heisenberg Uncertainty Principle.
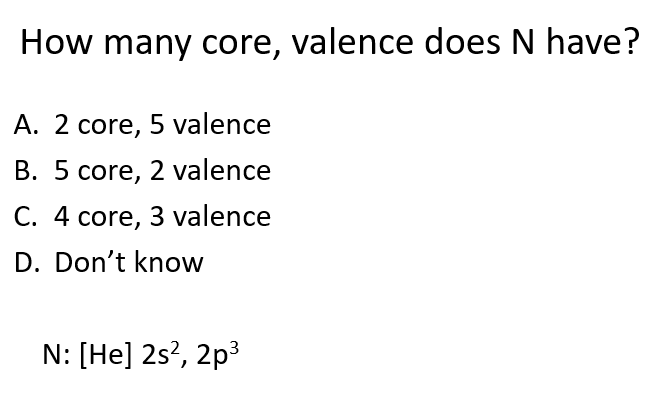
A
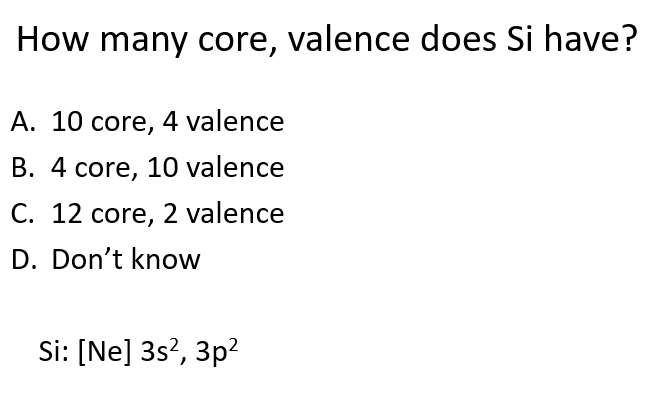
A

A
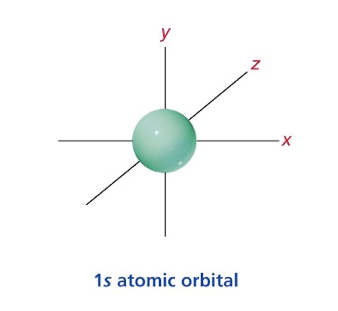
What does an S orbital(s) look like?
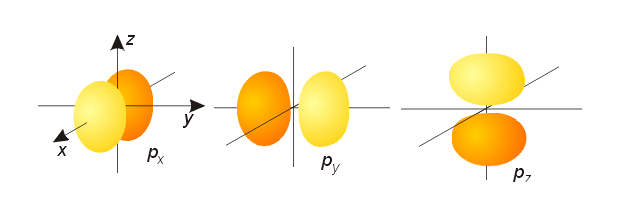
What does a P orbital(s) look like?
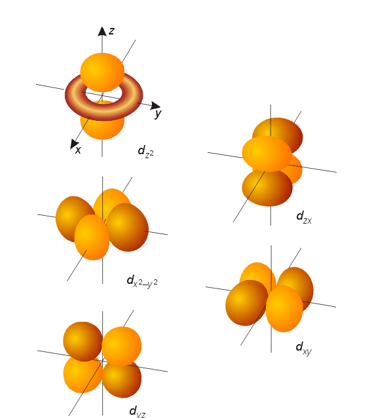
What does a D orbital(s) look like?
What are core electrons, and what role do they play?
Core electrons are low in energy, close to the nucleus, and within the closed shell. They are stable and do not participate in reactions.
How do you identify core electrons?
Identify core electrons using the last noble gas (group 18, e.g., Ne or Xe) and any full d shell (for transition metals).
What are valence electrons, and why are they important?
Valence electrons are higher in energy and outside the closed shell. These electrons determine an atom's reactivity.

B
The atomic radius of lithium is smaller than that of sodium because lithium has fewer electron shells. Lithium’s valence electron is in the second energy level (n=2), while sodium’s is in the third energy level (n=3). As a result, sodium has a larger atomic radius due to increased shielding and reduced effective nuclear attraction on the outermost electron.
Does atomic radius increase or decrease down a group?
Atomic radius increases down a group because electrons are added to higher energy levels, which are farther from the nucleus, and the increased shielding from inner electrons reduces the pull on outer electrons.
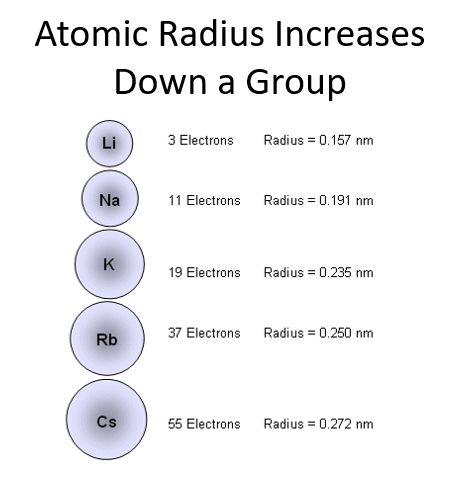

A
The atomic radius of lithium is larger than that of neon because neon has more protons, creating a stronger effective nuclear charge that pulls its electrons closer to the nucleus. Although both elements have electrons in the second energy level, neon’s additional protons cause a greater attraction, making its atomic radius smaller than lithium’s.
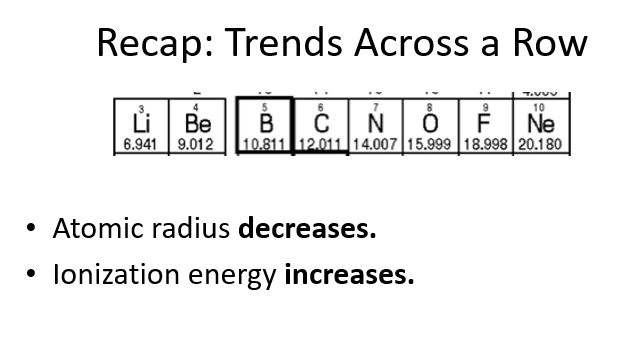
Does atomic radius increase or decrease across a row?
Atomic radius decreases across a row because the number of protons increases, which pulls the electrons closer to the nucleus, reducing the size of the atom.
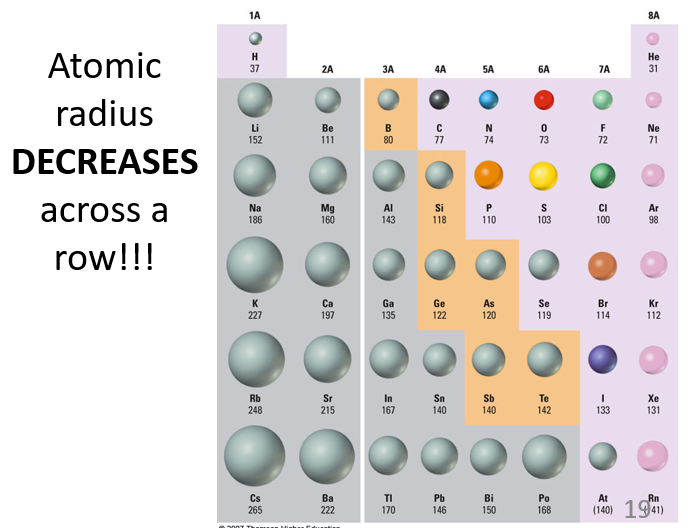
What factors determine the size of an atom?
The size of an atom depends on the balance between the attraction of protons and electrons and the repulsions between the electrons.
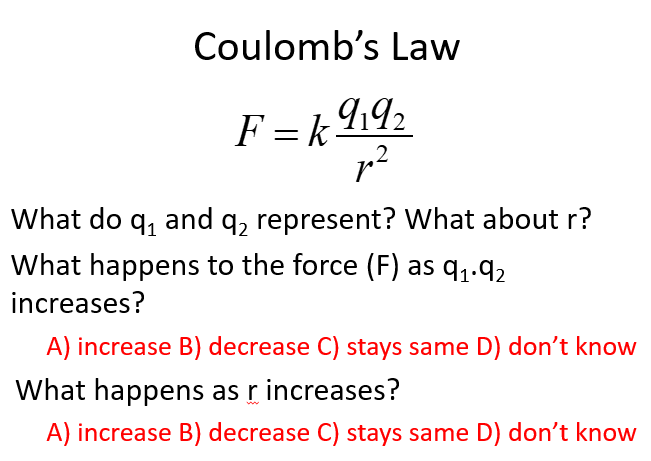
as q1q2 (how large a charge is) increases, force = increases
as R (distance between charges) increases, force = decreases
How does Coulomb’s law explain the size of an atom?
Coulomb’s law explains both the attractions between the protons and electrons, and the repulsions between the electrons, affecting the overall size of the atom.
What does the atomic radius represent?
The atomic radius represents the point where the forces of attraction between the electrons and protons are equal to the forces of repulsion between the electrons.
What is effective nuclear charge, and how is it calculated?
Effective nuclear charge is the net positive charge experienced by valence electrons. It’s calculated by subtracting the number of core electrons from the total number of protons.
Example: For carbon ([He] 2s²2p²), the effective nuclear charge is 6 protons - 2 core electrons = 4. Each valence electron feels an attraction from 4 protons.
![<p>Effective nuclear charge is the net positive charge experienced by valence electrons. It’s calculated by subtracting the number of core electrons from the total number of protons.</p><p>Example: For carbon ([He] 2s²2p²), the effective nuclear charge is 6 protons - 2 core electrons = 4. Each valence electron feels an attraction from 4 protons.</p>](https://knowt-user-attachments.s3.amazonaws.com/6a7e4207-3d52-4a36-814f-7878f4a983e6.png)
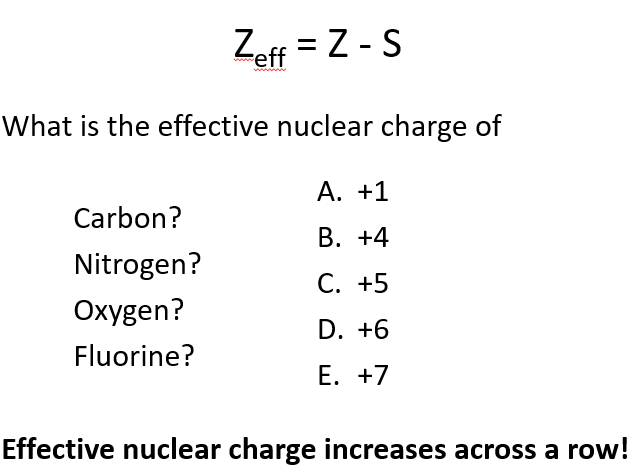
Carbon: +4
Nitrogen: +5
Oxygen: +6
Fluorine: +7
Why do atoms with a high effective nuclear charge hold on to their electrons tightly?
Atoms with a high effective nuclear charge have a stronger attraction between the electrons and the nucleus, causing the electrons to be more tightly held. This is why atomic radius decreases across a row.
What is an ion?
An ion is an atom in which electrons have been added or removed.

B
When an electron is removed from an atom, the atom becomes a positively charged ion (cation). This happens because electrons are negatively charged, and removing one reduces the total negative charge while the number of protons remains the same. As a result, the ion has more protons than electrons, giving it an overall positive charge.

A
Lithium has a larger atomic radius than Li+ because removing an electron reduces electron-electron repulsion, allowing the remaining electrons to be pulled closer to the nucleus. Since Li+ has the same number of protons but fewer electrons, the stronger effective nuclear charge results in a smaller radius.
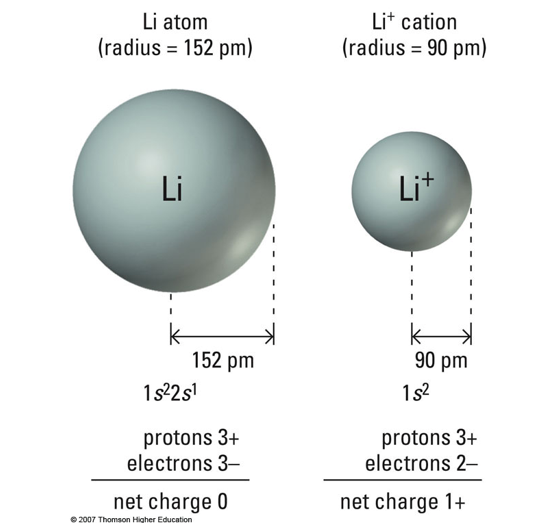

C
When an electron is added to an atom, the atom becomes a negatively charged ion (anion). This happens because electrons carry a negative charge, and adding one increases the total negative charge while the number of protons stays the same. Since there are now more electrons than protons, the ion has an overall negative charge.

B
The radius of F- is larger than that of neutral fluorine because adding an electron increases electron-electron repulsion in the outer shell. Since the number of protons remains the same, the increased repulsion causes the electron cloud to expand, resulting in a larger ionic radius.
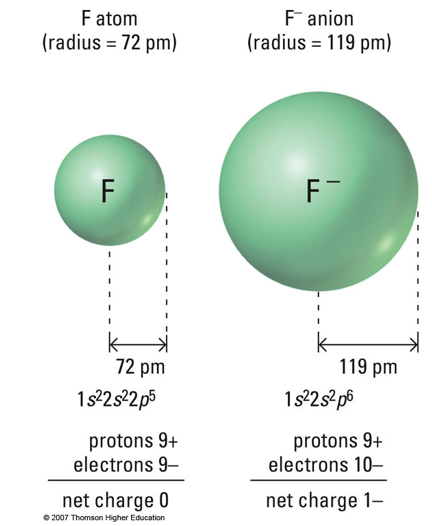

B
F- is larger than Na+ because F- has more electrons than protons, increasing electron-electron repulsion and expanding its electron cloud. In contrast, Na+ has more protons than electrons, causing a stronger nuclear attraction that pulls the remaining electrons closer. Additionally, Na+ has lost its outermost electron shell, making it much smaller than F-.
What is an isoelectronic series?
An isoelectronic series consists of ions or atoms that have the same electron configuration (e.g., 1s² 2s² 2p⁶) but different numbers of protons. As the charge on the nucleus increases, the attraction between the electrons and protons also increases.
What is ionization energy?
Ionization energy is the energy required to remove an electron from an atom in the gas phase.
Example: Li(g) → Li⁺(g) + e⁻ (Ionization energy = 520 kJ/mol)

B
It is easier to remove an electron from Na than from Li because sodium’s valence electron is in the third energy level (n=3), while lithium’s is in the second energy level (n=2). The increased shielding in sodium reduces the effective nuclear attraction on the outermost electron, making it easier to remove compared to lithium, where the valence electron is closer to the nucleus and more strongly attracted.
B
The ionization energy of Na is smaller than that of Li because sodium’s valence electron is in the third energy level (n=3), while lithium’s is in the second energy level (n=2). The increased distance and greater shielding in sodium reduce the effective nuclear attraction on the outermost electron, making it easier to remove. In contrast, lithium’s valence electron is closer to the nucleus and more strongly attracted, requiring more energy to remove.

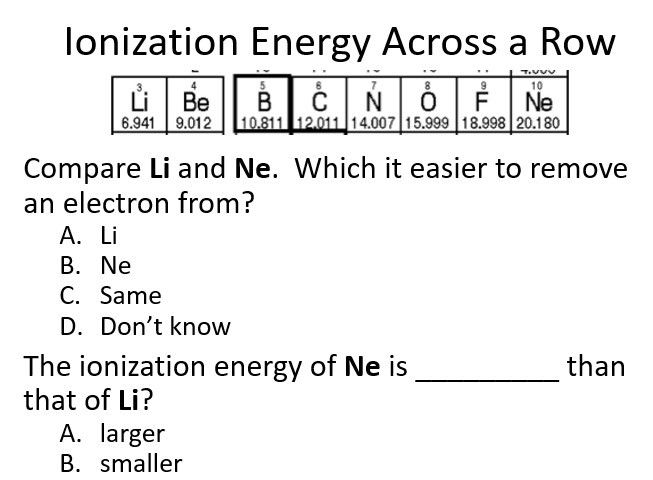
A
It is easier to remove an electron from Li than from Ne because lithium has only one valence electron in the second energy level, while neon has a full and stable octet. Lithium’s valence electron experiences less attraction to the nucleus, making it easier to remove. In contrast, neon’s full outer shell is very stable, requiring much more energy to remove an electron.
A
The ionization energy of Ne is larger than that of Li because neon has a full and stable octet, making it much harder to remove an electron. Neon also has more protons than lithium, increasing its nuclear charge and pulling its electrons closer. In contrast, lithium only has one valence electron, which is more weakly held, so less energy is required to remove it.
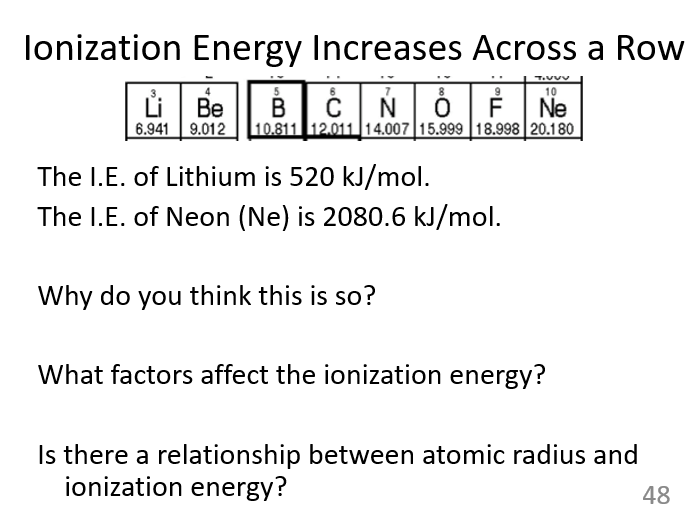
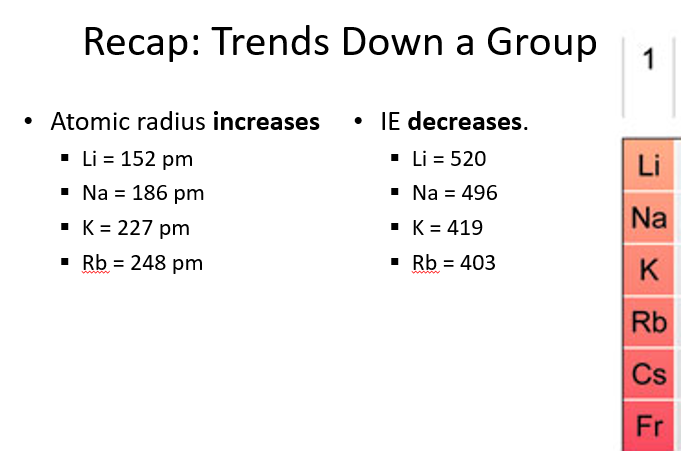
How are atomic radius and ionization energy related?
Atomic radius and ionization energy are inversely related—small atoms have higher ionization energies. This is caused by the same phenomenon: the effective nuclear charge.
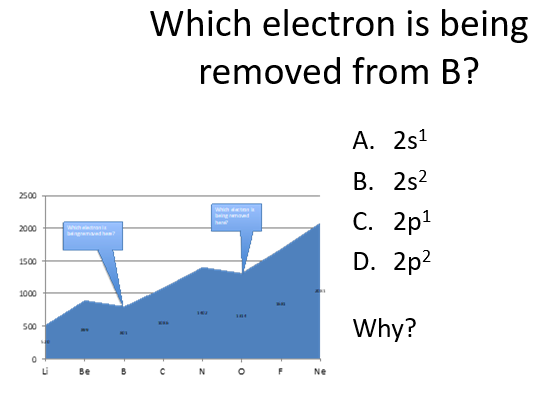
C
The electron being removed from B is the 2p1 electron because it is the highest-energy valence electron. Boron’s electron configuration is 1s2 2s2 2p1, and since ionization removes the outermost electron first, the 2p1 electron is removed because it is higher in energy and less tightly held than the 2s2 electrons.
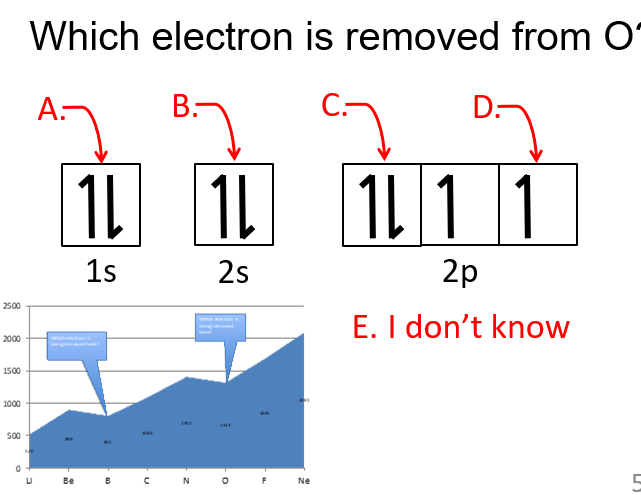
C
The electron being removed from oxygen is a 2p electron because it is the highest-energy valence electron. Oxygen’s electron configuration is 1s2 2s2 2p4, and since ionization removes the outermost electron first, one of the 2p electrons is removed because it is higher in energy and less tightly held than the 2s electrons.
Why is it easier to remove the lone electron from a p orbital (e.g., in group 13 elements like B)?
It is easier because the two s electrons are more tightly held to the nucleus and harder to remove, so the p electron is more easily removed.
Why is it easier to remove the first paired electron from group 16 (e.g., O, S)?
It is easier because there is slight repulsion when electrons pair up in an orbital, making the paired electron easier to remove.

C
The third ionization energy of Mg is the largest because it requires removing a core electron rather than a valence electron. Valence electrons are easier to remove because they are farther from the nucleus and experience weaker attraction. However, core electrons are closer to the nucleus, experience a stronger electrostatic attraction, and have less shielding, making them much harder to remove. As a result, much more energy is needed to overcome this strong attraction
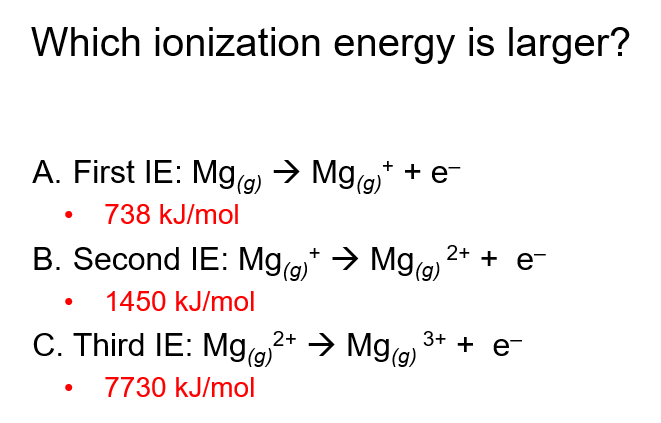
Why is there a huge jump in the 3rd ionization energy of Mg?
The huge jump occurs because the electron is being removed from the "core" rather than the valence shell, making it much harder to remove.
What factors affect ionization energy?
Ionization energies are affected by:
Size of the atom/ion: Smaller size → higher ionization energy.
Size of positive charges: Larger charge → larger ionization energy.
The shell the electron is removed from: Ionization energy of core electrons is higher than that of valence electrons.
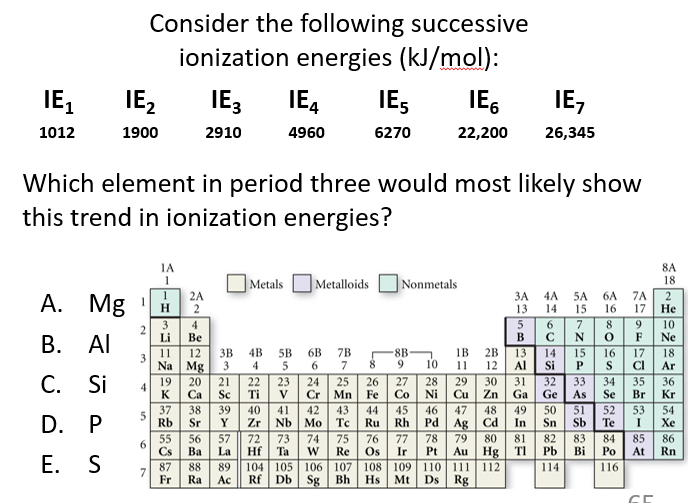
D
The element is P because of the large jump between IE5 and IE6. Phosphorus has five valence electrons, so the first five ionizations remove valence electrons. The sixth ionization removes a core electron, which is much harder to remove due to stronger nuclear attraction, causing the large increase in ionization energy.