Biochem Lec 12- Enzymes IV: Catalytic Mechanisms & Chymotrypsin
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
What is Chymotrypsin?
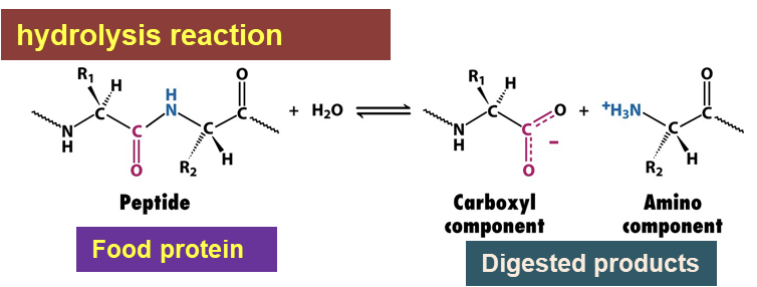
A digestive enzyme that is produced in the pancreas and is secreted into the small intestine
It aids in the digestion of food proteins by catalyzing the hydrolysis of peptide bonds
What are enzymes that breakdown proteins by hydrolyzing the peptide bonds called? (i.e. Chymotrypsin)
Proteases
What type of protease is Chymotrypsin and what does it do?
Endoprotease → cleaves peptide bonds inside the polypeptide chain
What is Chymotrypsin specific for?
It is highly specific for protein substrates based on the amino acid R-group on the amino terminal side of the cleaved peptide bond (scissile peptide bond)
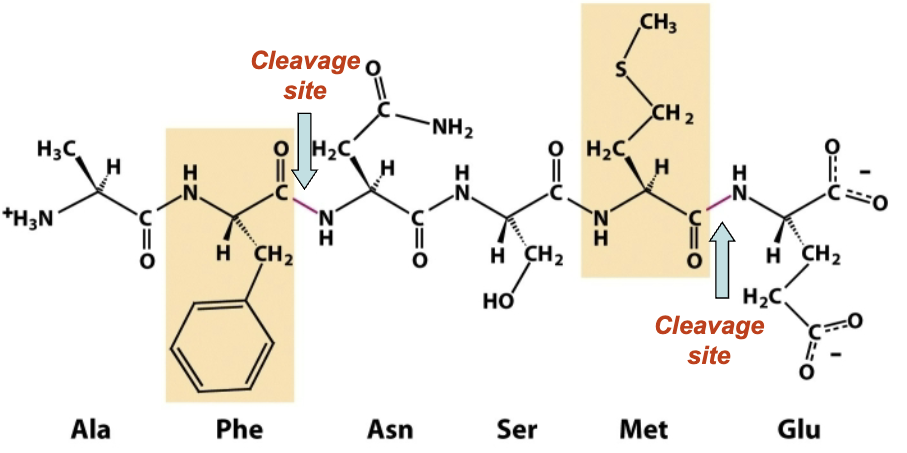
What peptide bonds does Chymotrypsin hydrolyze?
Peptide bonds on the carboxyl terminal side of aromatic (trp, phe, tyr) or methionine (met) residues
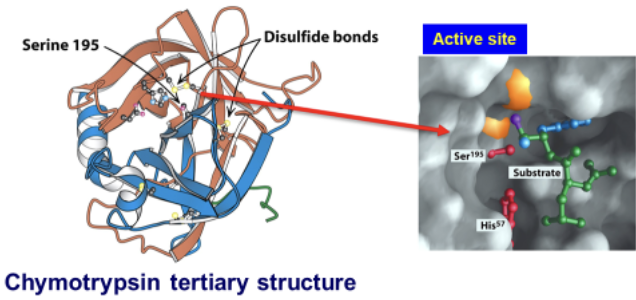
Chymotrypsin’s active site contains a special residue. What is it? Describe it.
Ser 195 → different from the other serine residues in the enzyme and is a highly reactive nucleophile
Enzymes with reactive serines are susceptible to what? What are they and what do they do?
organofluorophosphate neurotoxins → irreversibly inactivate these enzymes by covalent modification of highly reactive serines
Ex. diisopropylfluorophosphate (DIPF) and sarin
These compounds irreversibly inactivate these enzymes by covalent modification of highly reactive serines
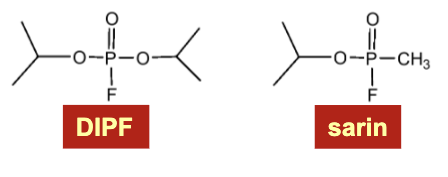
Why is serine 195 reactive?
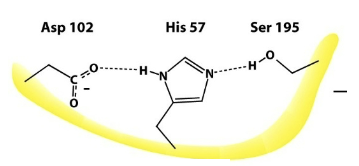
Because of two other active site amino acids: His57 and Asp102
What AA’s make up the “catalytic triad” of chymotrypsin?
Ser195, His57, and Asp102
What AA is Ser195 H-bonded to? What effect does this have on Ser195?
Ser195 is H-bonded to His57→ this increases the deprotonation of the Ser hydroxyl group, making it more strongly nucleophilic.
What property of His57 allows it to more readily promote the removal of the Ser195 -OH proton?
It is more basic than normal histidines→ pKa is >7.5
What AA does Asp102 stabilize? How does it stabilize this AA? What effect does this have on the AA?
Asp102 stabilizes His57 because the neg. charge on Asp102 forms ionic interactions with the pos. charge on His57. This increases His57’s properties as a base→ stabilizes the pos. charge on His57 when it accepts a proton from Ser195→ increases basicity by stabilizing His57, making it a stronger proton acceptor
In what class of enzymes is the catalytic triad?
Serine proteases and hydrolases
Serine proteases and hydrolases like Chymotrypsin have three conserved features in their active sites. What are they?
The catalytic triad
The S1 pocket
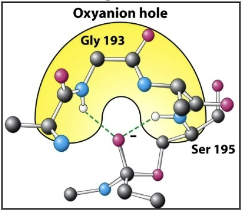
The oxyanion hole
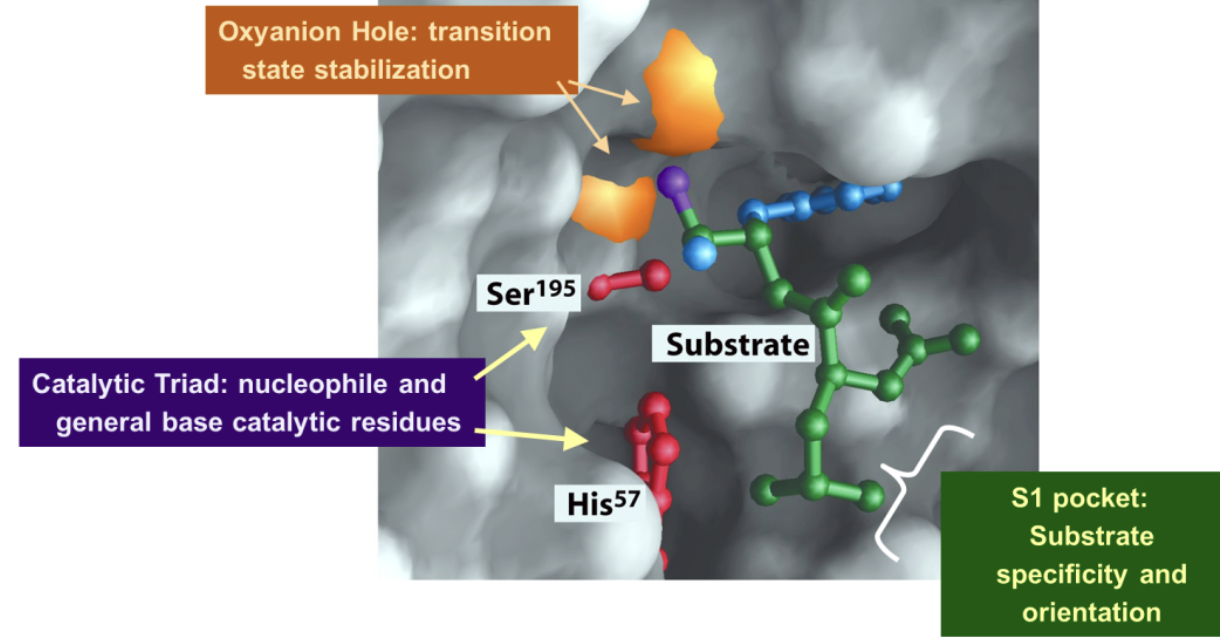
What is the role of the catalytic triad during catalysis?
Catalyzes the hydrolysis of the peptide bond in two steps
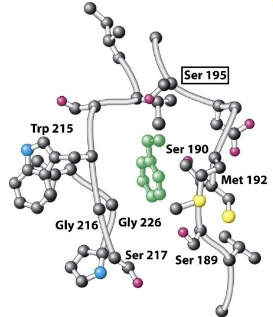
What is the role of the S1 pocket during catalysis?
Binds and positions the peptide substrate so that the catalytic triad can work
What is the role of the oxyanion hole during catalysis?
Stabilizes the transition state via hydrogen bonding during the reaction
The chymotrypsin reaction is an example of what type of catalysis?
Covalent catalysis
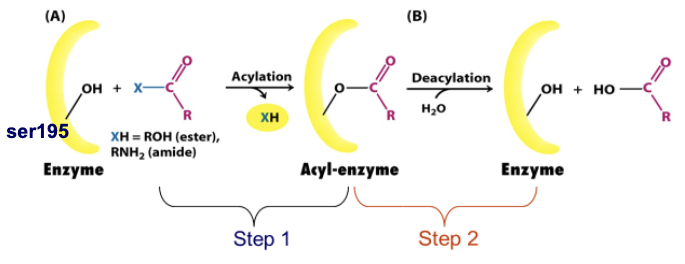
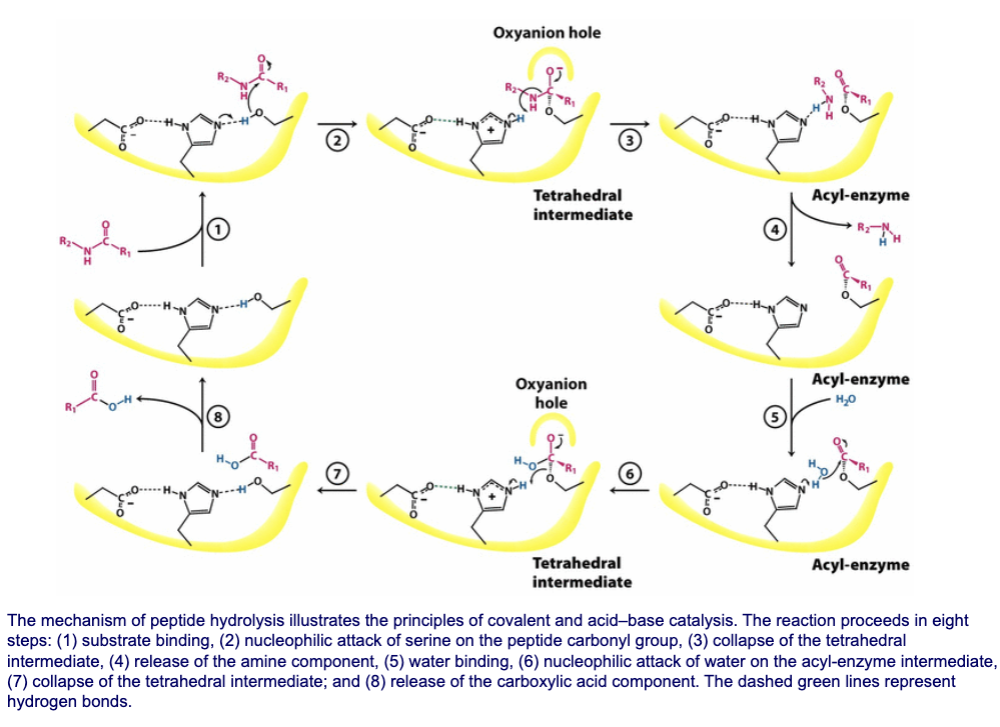
The chymotrypsin reaction proceeds through ___ steps. What are these steps?
2
Formation of acyl enzyme intermediate
Hydrolysis of acyl enzyme intermediate
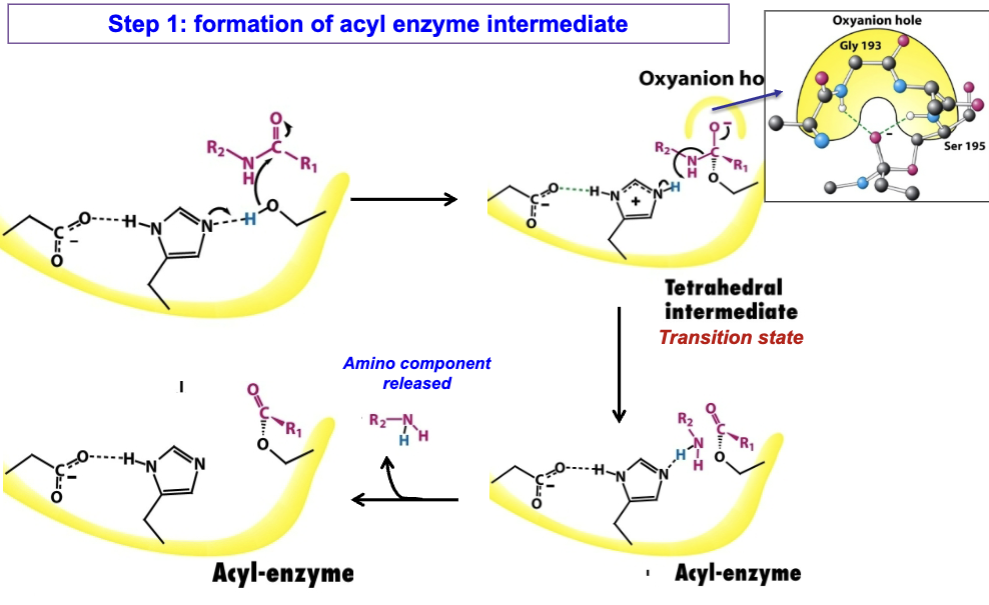
What occurs during the first step of the chymotrypsin reaction?
Reactive serine cleaves peptide bond and releases the amino component. The carboxyl component remains attached to enzyme as an “acyl enzyme” intermediate
Substrate binding
Peptide substrate sits in active site
Its P1 aromatic side chain fits into S1 pocket→ aligns peptide bond being cleaved next to Ser195
Activation of Ser195/Nucleophilic attack
His57 acts as base→ accepts proton from -OH of Ser195→ made possible by Asp102 stabilizing His57→ makes it more basic
Deprotonated Ser195 (strong alkoxide ion) attacks carbonyl of peptide bond
Forms tetrahedral intermediate at carbonyl carbon (stab. by oxyanion hole)
Collapse of tetrahedral intermediate
Oxyanion reforms carbonyl double bond, forcing peptide bond to break
Release of amine component
His57 donates a proton to leaving group (the amine end of the peptide)
Amine product diffuses away, leaving the acyl-enzyme intermediate
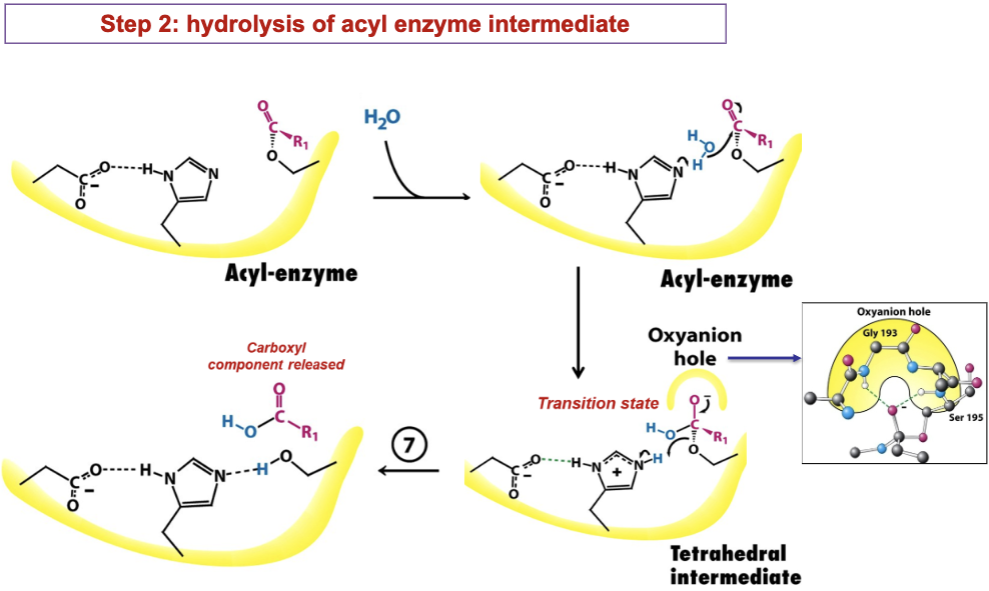
What happens during the second step of the chymotrypsin reaction?
Water binding
A water molecule enters active site and takes position once held by amine product
His57 orients water molecule for next step
Nucleophilic attack of water on the acyl-enzyme intermediate
His57 accepts proton from water→ forms hydroxide anion
Hydroxide anion attacks carbonyl carbon of acyl-enzyme intermediate
Forms second tetrahedral intermediate (stab. by oxyanion hole)
Collapse of second tetrahedral intermediate
Second tetrahedral intermediate collapses, reforming carbonyl double bond
Bond between Ser195 and substrate breaks
His57 donates proton back to Ser195, reforming its -OH
Release of carboxylic acid component
The carboxylic acid product (the C-terminal fragment of the substrate) is released
Enzyme is fully regenerated/ready for another catalytic cycle
Full chymotrypsin mechanism
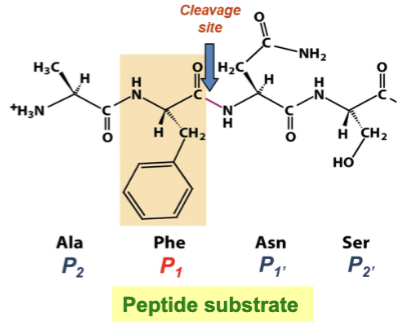
What AA is chymotrypsin highly specific for?
The AA residue found on the amino terminal side of the peptide bond (P1 residue)
Substrate specificity is accomplished by what?
The S1 pocket→ S1 pocket binds the P1 and positions substrate
What is the chymotrypsin S1 pocket lined with? How does this accommodate substrate binding?
Hydrophobic residues→ accommodates substrate binding by interacting with the aromatic R-groups of the P1 amino acid residue.
Different proteases and hydrolases contain a common catalytic feature of the catalytic triad, reactive Ser, and oxyanion hole. What is this?
Specificity for different substrates achieved by different chemical complementarity of the S1 pocket.
What is the location/function/P1 specificity of chymotrypsin?
Location→ small intestine
Function→ protein/substrate digestion
P1 specificity→ Aromatic AA’s and Methionine
What is the location/function/P1 specificity of trypsin?
Location→ small intestine
Function→ protein substrate digestion
P1 specificity→ positively charged AA’s (Arg and Lys)
What is the location/function/P1 specificity of elastase?
Location→ pancreas and neutrophils (type of white blood cell)
Function→ breakdown of connective tissue
P1 specificity→ small AA’s (Ala, Ser, Gly)
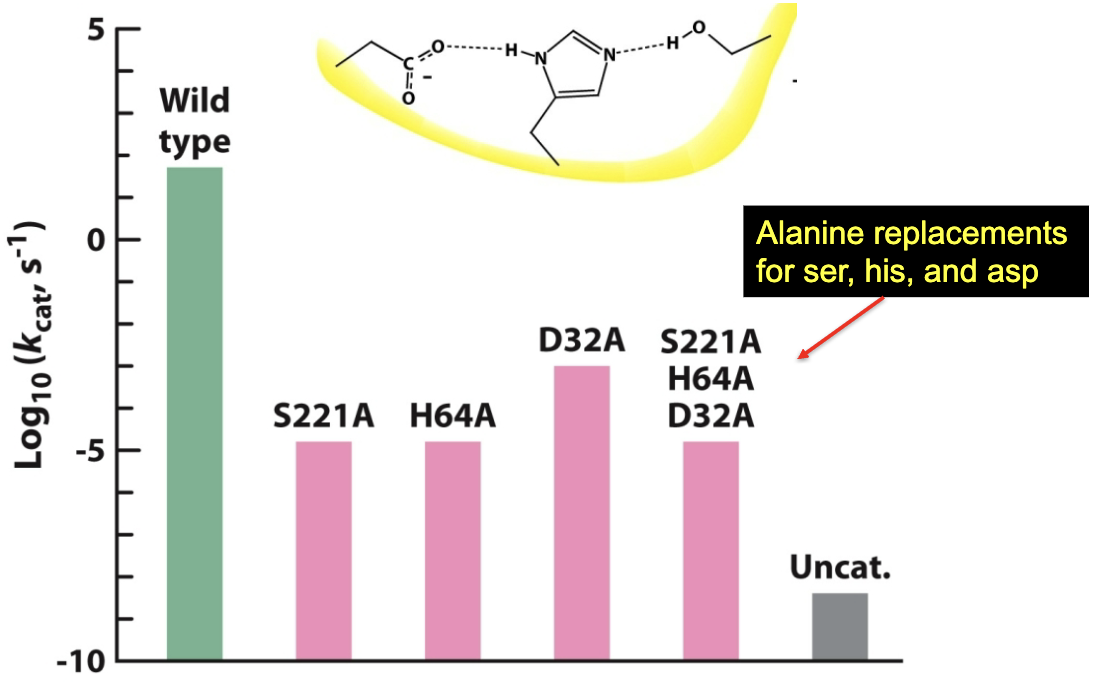
Site-directed mutagenesis and enzyme kinetics reveal what?
The critical nature of each residue of the active site catalytic triad. It shows that mutating any of the three residues drastically reduces catalytic efficiency→ triad is necessary for efficient catalysis.