Biochemistry - Module II
1/176
Earn XP
Description and Tags
5, 7, 8, 9
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
177 Terms
How is penguin mate recognition similar to molecular recognition?
Like penguins distinguishing their mates among many similar birds, proteins can recognize and bind specific molecules even among many similar alternatives.
What is molecular recognition?
The ability of specific molecules (e.g., proteins and ligands) to bind to one another in the presence of many alternatives.
Write the general reaction for molecular binding.
R + L ⇌ RL (Receptor + Ligand ⇌ Receptor-Ligand Complex)
Why is binding fundamental in biochemistry?
It enables organization within crowded cells, allowing macromolecules to selectively bind partners and form pathways and assemblies.
What does L1/2 represent?
The ligand concentration at which half of the receptor is bound (half-maximal binding). It inversely measures binding affinity.
What happens to binding as ligand concentration increases?
At low affinity, few complexes form; at high affinity, receptors become saturated with ligand as concentration rises.
Define Kd (dissociation constant).
Kd = [R][L]/[RL]; it describes the equilibrium between free receptor, free ligand, and the receptor-ligand complex.
How are Kd and L1/2 related?
Kd = L1/2 at half saturation of receptors.
How is specificity quantified?
By comparing Kd values for different ligands—the lower the Kd, the tighter the binding.
What is the typical range of Kd values for proteins?
From millimolar (weak binding) to femtomolar (extremely tight binding).
Give an example of extremely tight protein–ligand binding.
Avidin and biotin (Kd ≈ 10⁻¹⁵ M).
What is the tightest known protein–protein interaction?
RNase 1 bound by its inhibitor RI (Kd = 3.5 × 10⁻¹⁷ M).
Why is RNase 1–RI so tight?
It has an enormous interaction surface with many favorable noncovalent interactions.
Where is myoglobin found and what is its role?
In muscle tissue; facilitates oxygen diffusion and serves as an oxygen reserve.
Where is hemoglobin found and what is its role?
In red blood cells; transports oxygen from lungs to tissues and carries CO₂ and H⁺ back to lungs.
What are the forms of these proteins regarding oxygen?
“Deoxy” (oxygen-free) and “Oxy” (oxygen-bound).
What enables myoglobin and hemoglobin to bind oxygen?
The heme prosthetic group.
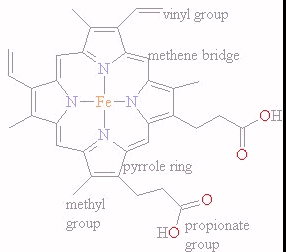
Describe the heme group.
Made of protoporphyrin (four pyrrole rings) plus a central Fe²⁺ ion.
Fe²⁺ is bonded to four pyrrole nitrogens and can form two additional coordination bonds.
What binds to the fifth coordination site?
The imidazole ring of a proximal histidine residue.
What binds to the sixth coordination site?
Oxygen (in oxyhemoglobin/oxymyoglobin).
What is the function of the distal histidine?
It stabilizes O₂ binding with a hydrogen bond, blocks larger molecules, prevents Fe²⁺ oxidation, and reduces CO affinity.
Why does CO bind less strongly than O₂ in globins?
CO’s binding orientation prevents the stabilizing H-bond with the distal histidine.
What shape is the myoglobin oxygen-binding curve?
Hyperbolic (P₅₀ ≈ 2 torr).
What shape is the hemoglobin oxygen-binding curve?
Sigmoid (S-shaped) (P₅₀ ≈ 26 torr).
What does the sigmoid shape indicate?
Cooperativity—binding of O₂ at one site increases the likelihood of binding at other sites.
Define allosteric protein.
A protein whose function at one site is affected by binding at another site; usually multisubunit.
How many subunits does hemoglobin have?
Four: two α chains and two β chains (α₁β₁ and α₂β₂ dimers).
What happens to hemoglobin quaternary structure upon oxygen binding?
α₁β₁ and α₂β₂ dimers rotate 15°; hemoglobin shifts from T (tense) to R (relaxed) state.
What does the concerted model propose?
Hemoglobin exists only in T or R states; oxygen binding shifts the equilibrium toward R.
What does the sequential model propose?
Binding of oxygen at one site increases the affinity of neighboring sites without full conversion to R.
Which model best fits hemoglobin?
Neither purely—hemoglobin shows a mixture of both models.
What causes sickle cell hemoglobin (HbS)?
A single amino acid substitution: valine for glutamate at position 6 of β chains.
Why does this cause sickling?
Valine’s hydrophobic side chain promotes aggregation of deoxygenated HbS molecules into fibers.
What is the vicious cycle of sickling?
Blocked vessels cause low oxygen, which promotes more deoxygenated HbS, leading to more sickling and damage.
What new treatment approach has shown promise for sickle cell anemia?
CRISPR-Cas9 gene editing to induce γ chain expression and reverse pathology.
Who was Roland Scott and why is he important?
A pediatrician and sickle cell researcher at Howard University. His 1948 paper on sickle cell incidence in infants changed perceptions of the disease and led to newborn screening programs.
What was Roland Scott’s biggest legislative success?
Advocacy for the National Sickle Cell Control Act of 1972, which improved research and care.
Why is Roland Scott’s contribution notable?
He demonstrated sickle cell disease affects multiple ancestries, not just African ancestry, and championed patient care and awareness.
What is the relationship between L ½ and tight binding?
The tigher the binding, the lower the L ½ value
The looser the binding, the higher the L ½ value
If a certain drug has a 1 nm Kd what does that mean?
This drug has a higher binding affinity due to its low Kd. This is ideal.
If a certain drug has a 1 micro meter Kd what does that mean?
This drug has a lower binding affinity due to its high Kd. This is not ideal.
Why do drugs need to have a high binding affinity and low dissociation?
To achieve and maintain sufficient target occupancy, leading to a stronger, more prolonged, and more selective therapeutic effect with fewer side effects.
High affinity means the drug binds tightly to its intended target, and low dissociation means it stays bound for a longer time
Which affinity is highest? 10-15 or 10-17
10-17 is the higher affinity.
Why is pressure used to measure fractional saturation?
A gas's partial pressure is directly proportional to its concentration when it is dissolved in a liquid, following Henry's Law
What is p50?
Pressure at which 50% of binding sites are occupied (same as L1/2)
What does kinetics study in a reaction?
The rate (speed) at which reactants become products; how concentrations change over time.
What does thermodynamics study in a reaction?
The spontaneity and equilibrium of a reaction; compares initial and final states.
What are the key constants for kinetics and thermodynamics?
Kinetics uses rate constants (k); thermodynamics uses Keq, ΔG, ΔH, and ΔS.
How do temperature and catalysts affect kinetics vs. thermodynamics?
Both are affected by temperature; catalysts change reaction rates (kinetics) but not spontaneity (thermodynamics).
How is reaction velocity (rate) defined?
Rate of disappearance of A or appearance of P

What defines a first-order reaction?
Rate proportional to one reactant’s concentration: v=k[A]; units of k = s⁻¹.
What defines a second-order reaction?
Rate proportional to either two molecules of A or A + B: v = k[A]² or v = k[A][B]; units of k = M-1s-1
What defines zero-order and third-order reactions?
Zero-order: rate independent of [reactant]. Third-order: rate proportional to the cube of concentrations or combination to equal 3.
How can you distinguish first, second, third, and zero order reactions?
By how rate changes with concentration:
First order: rate ∝ [A]
Second order: rate ∝ [A]^2 or [A][B]
Third order: rate ∝ [A]^3 or combinations
Zero order: rate constant regardless of [A]
Who developed the Michaelis–Menten model?
Leonor Michaelis and Maud Menten.
What does the Michaelis–Menten equation describe?
The initial velocity of an enzyme-catalyzed reaction as a function of substrate concentration.
What is initial velocity (V₀)?
The reaction rate measured soon after the reaction begins, before significant product accumulates.
What is Vmax?
The maximal rate of an enzyme-catalyzed reaction when enzyme is saturated with substrate.
What is KM?
Substrate concentration at which initial velocity is half of the max velocity, V₀ = Vmax/2; reflects enzyme affinity for substrate.
What is the Lineweaver–Burk plot?
A double-reciprocal plot (1/V₀ vs. 1/[S]) used to determine KM and Vmax from a straight line.
How does KM relate to substrate concentration in vivo?
Many enzymes have KM close to their physiological substrate concentration, making them sensitive to changes (elasticity).
What is the turnover number (kcat)?
The number of substrate molecules converted to product per enzyme per unit time at saturation; kcat = Vmax / [E]T.
What does kcat/KM measure?
Catalytic efficiency; rate constant for enzyme-substrate interaction at low [S]; also called specificity constant.
What are typical KM and kcat examples?
Carbonic anhydrase has KM ~8000 M and kcat ~600,000 s⁻¹; lysozyme KM ~6 M and kcat ~0.5 s⁻¹.
What defines sequential (single-displacement) reactions?
All substrates bind enzyme before product release; ternary complex forms. Can be ordered or random.
What defines double-displacement (ping-pong) reactions?
One or more products released before all substrates bind; enzyme forms a temporary intermediate.
What do allosteric enzymes control?
The committed (first irreversible) step of metabolic pathways; they act as catalysts and information sensors.
How are allosteric enzymes regulated?
By feedback inhibition—products bind to a regulatory (non-active) site to modulate activity.
How do velocity curves differ between Michaelis–Menten and allosteric enzymes?
M–M enzymes: hyperbolic curve; allosteric enzymes: sigmoidal curve due to cooperativity.
What is the concerted model of allostery?
Enzyme exists in T (tense) and R (relaxed) states; all subunits switch together (symmetry rule). Substrate binds R more easily; binding shifts equilibrium toward R.
What is the physiological significance of cooperativity?
Enzyme activity switches on or off within a narrow substrate range (threshold effect), making it more sensitive than M–M enzymes.
How do positive and negative effectors modulate T⇌R equilibrium?
Positive effectors stabilize R, lower substrate threshold; negative effectors stabilize T, raise threshold.
What is the difference between heterotropic and homotropic effects?
Heterotropic: effector and substrate are different molecules; homotropic: effect due to multiple binding of the same substrate.
What is the sequential model of allostery?
Subunits change conformation individually; allows for negative cooperativity where one binding event reduces affinity at other sites.
What are ensemble studies?
Traditional experiments on solutions containing millions of enzyme molecules; report average properties.
What are single-molecule experiments?
New methods that observe one enzyme at a time, revealing transient states or rare events not visible in ensemble averages.
What is the initial velocity for an enzyme with Vmax = 100 moles/sec, KM = 5000 M, and [S] = 100 M?
1.96 moles/sec (calculated from the Michaelis–Menten equation).
In a metabolic pathway, how can you identify the allosteric enzyme?
It catalyzes the first irreversible (committed) step leading uniquely to the product; often has large negative ΔG.
What’s an analogy for kinetics vs. thermodynamics?
Kinetics = speed limit, Thermodynamics = destination.
Kinetics tells you how fast you get somewhere; thermodynamics tells you if it’s even worth going.
How do catalysts fit into this analogy for kinetics vs. thermodynamics?
A catalyst is like building a faster road or removing traffic—it doesn’t change the destination (ΔG) but makes the trip faster (k).
How can you picture first-order vs. second-order reactions?
First-order = one-person job (like one student copying notes).
Second-order = two-person handshake (two people must meet for reaction to occur).
How to remember zero-order reactions?
Think of a fully booked restaurant kitchen — no matter how many orders come in, output is maxed out, so rate stays constant.
What’s an analogy for KM?
KM is like the “crowd size” needed for a concert to be at half capacity.
Low KM = fills up quickly (high affinity)
high KM = needs more people (substrate) to reach half-max.
How to picture Vmax?
Vmax is like every seat in a theater being filled — once all seats (active sites) are taken, adding more people (substrate) won’t speed up seating.
What’s an easy way to remember the Michaelis–Menten curve?
Think of a roller coaster ramping up quickly then leveling off; initial steep slope = velocity increases with [S], plateau = Vmax.
Mnemonic for the Lineweaver–Burk plot?
“1 over everything”: plot 1/V vs 1/[S] to straighten the curve. Left intercept = -1/KM, top intercept = 1/Vmax.
Analogy for kcat (turnover number)?
kcat = number of pizzas a chef can bake per hour when the kitchen is fully stocked.
Analogy for kcat/KM (catalytic efficiency)?
kcat/KM = how good the chef is at turning ingredients into pizza when ingredients are scarce — combines speed and binding skill.
How to remember sequential vs. ping-pong reactions?
Sequential = everyone at the table at once (both substrates present before meal starts).
Ping-pong = tag-team (one leaves before the other enters, like ping-pong ball bouncing).
Analogy for allosteric vs Michaelis–Menten enzymes?
M–M enzymes = dimmer switch (smooth hyperbolic response).
Allosteric enzymes = light switch (sigmoidal “on/off” threshold effect).
How to remember T and R states?
Tense (T) = tight fists (inactive)
Relaxed (R) = open hands (active)
Substrate binds open hands more easily.
Mnemonic for positive and negative effectors?
“Positive = Pushes to R” (active); “Negative = Ties to T” (inactive).
How to picture cooperativity?
Like a team clapping in sync: once one starts, others join rapidly — sharp, sigmoidal increase.
Analogy for ensemble vs. single-molecule studies?
Ensemble = “average class GPA” (everyone’s grades combined)
single-molecule = “individual student’s grade” (details of one).
How to decide which enzyme is allosteric in a pathway?
Look for the first “point of no return” (committed step) — like the toll booth before a one-way bridge. Once you cross, you’re committed.
Which term is MOST appropriate to explain an enzyme binding to its substrate?
induced fit
A tightly bound cofactor might be called a(n):
prosthetic group
Choose the CORRECT statement. Enzymes:
are very specific
Enzymes that transfer electrons are called:
oxidoreductases
Riboflavin is a water-soluble organic substance that is not synthesized by humans. Metabolically, it is converted into a substance called flavin adenine dinucleotide, which is required by succinate dehydrogenase. Choose the correct statement.
Flavin adenine dinucleotide is a coenzyme.