Module 1 Problem Set: Measurement, Matter, and Atomic Theory
1/89
Earn XP
Description and Tags
Chemistry
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
90 Terms
What are the 3 domains of chemistry?
Macroscopic, Microscopic, and Symbolic Domain
Describe the Macroscopic Domain
large enough to be sensed directly by human sight or touch
Describe the Microscopic Domain
visited in the imagination (or visible through microscopes, etc)
Describe the Symbolic Domain
specialized language used to represent components of the macroscopic and microscopic domains
Classify the following as one of the 3 domains of chemistry: H2O(l)
symbolic
Classify the following as one of the 3 domains of chemistry: a lead pencil
macroscopic
Classify the following as one of the 3 domains of chemistry: a water bottle
macroscopic
Classify the following as one of the 3 domains of chemistry: a carbon atom
microscopic
Classify the following as one of the 3 domains of chemistry: Cu(s)
symbolic
Classify the following as one of the 3 domains of chemistry: an electron
microscopic
What are the 3 basic parts of a measurement?
a number, a unit, and indication of uncertainty
Write the correct symbol and the property measured: second
s, time
Write the correct symbol and the property measured: meter
m, length
Write the correct symbol and the property measured: kelvin
K, temperature
Write the correct symbol and the property measured: kilogram
kg, mass
Write the correct symbol and the property measured: kg per cubic meter
kg/m3, density
Write the correct symbol and the property measured: mole
mol, amount of substance
Write the correct symbol and the property measured: cubic meter
m3, volume
What is the mass of a lead cylinder that has a volume of 54.2 cm3 and a density of 11.34 g/cm3?
Equation:
Equation with values substituted:
Answer (with correct units and to 3 significant figures):
Equation: m = d × V
Equation with values substituted: m= 11.34g/cm3 x 54.2cm3
Answer (with correct units and to 3 significant figures): 615 g
What is the difference between an exact number and an uncertain measurement?
exact number - derived from counting
uncertain measurement - derived from a measurement other than counting and subject to uncertainty due to limitations of the measurement process used
Classify the following as either an exact number or an uncertain measurement: There are 4 quarts in 1 gallon.
exact
Classify the following as either an exact number or an uncertain measurement: The volume of the liquid is 12.3 mL.
uncertain
Classify the following as either an exact number or an uncertain measurement: There are 1000 g in 1 kg.
exact
Classify the following as either an exact number or an uncertain measurement: The ball has a mass of 10 g.
uncertain
Classify the following as either an exact number or an uncertain measurement: There are 10 pumpkins in the field.
exact
Determine how many significant figures are in the number: 0.01354
4
Determine how many significant figures are in the number: 1300
2
Determine how many significant figures are in the number: 520.3
4
Determine how many significant figures are in the number: 0.0430
3
Determine how many significant figures are in the number: 102.0
4
Perform the following calculations and report your answers to the correct number of significant figures: 11.2 + 3.45 + 7.845 + 8.9 = ______
31.4
Perform the following calculations and report your answers to the correct number of significant figures: 321/52 = ______
6.2
Perform the following calculations and report your answers to the correct number of significant figures: 113.5-6.22 = ______
107.3
Perform the following calculations and report your answers to the correct number of significant figures: 63.5 × 12 = ______
760
A metal cylinder was submerged in a graduated cylinder partially filled with water. The initial volume of water in the graduated cylinder before the metal cylinder was added was 10.5 mL. After the metal cylinder was submerged, the volume in the graduated cylinder was 15.6 mL. The mass of the metal cylinder is 98.4 grams.
1. What is the density of the metal cylinder?
Equation:
Equation with known values substituted:
Answer (with correct units and to 3 significant figures):
2. Given the following information, what is the metal rod made out of?
Metal | Density (g/cm3) |
tin | 7.29 |
tungsten | 19.3 |
zinc | 7.14 |
d = m/V
d= 98.4/ (15.6 mL - 10.5 mL)
19.3 g/mL (or g/cm3
Tungsten
Use dimensional analysis and the conversion factors below to perform the following unit conversion. You must show the setup of the calculation and the final answer to receive full credit. You do NOT need to show your intermediate work.
1 m = 1.0936 yd | 1 L = 1.0567 qt | 1 kg = 1000 g |
1 km = 0.62137 mi | 1 qt = 0.94635 L | 1 g = 100 cg |
1 mi = 1609.3 m | 1 kg = 2.2046 lb | 1 g = 1000 mg |
1 in = 2.54 cm | 1 lb = 453.59 g | 1 L = 1000 mL |
A girl ran 3.56 miles (mi). How many meters (m) did she run?
3.56 mi x (1609.3 m/ 1 mi) = 5730 m
Use dimensional analysis and the conversion factors below to perform the following unit conversion. You must show the setup of the calculation and the final answer to receive full credit. You do NOT need to show your intermediate work.
1 m = 1.0936 yd | 1 L = 1.0567 qt | 1 kg = 1000 g |
1 km = 0.62137 mi | 1 qt = 0.94635 L | 1 g = 100 cg |
1 mi = 1609.3 m | 1 kg = 2.2046 lb | 1 g = 1000 mg |
1 in = 2.54 cm | 1 lb = 453.59 g | 1 L = 1000 mL |
The boy drank 2 quarts of iced tea. How many milliliters (mL) of tea did he drink?
2 qts x (0.94635 L/ 1 qt) x (1000 mL/ 1 L)= 1890 mL
Use dimensional analysis and the conversion factors below to perform the following unit conversion. You must show the setup of the calculation and the final answer to receive full credit. You do NOT need to show your intermediate work.
1 m = 1.0936 yd | 1 L = 1.0567 qt | 1 kg = 1000 g |
1 km = 0.62137 mi | 1 qt = 0.94635 L | 1 g = 100 cg |
1 mi = 1609.3 m | 1 kg = 2.2046 lb | 1 g = 1000 mg |
1 in = 2.54 cm | 1 lb = 453.59 g | 1 L = 1000 mL |
The cube weighs 2.75 pounds (lb). How many centigrams (cg) is the cube?
2.75 lbs x (453.59 g/ 1 lb) x (100 cg/ 1 g) = 1.25x105 cg or 125,000 cg
Use the following conversion factors to convert between the different temperature scales below.
Equations and calculations with values substituted:
Answer (with correct units and to 3 significant figures):
25.2 °C = ______°F
77.4 F
Use the following conversion factors to convert between the different temperature scales below.
Equations and calculations with values substituted:
Answer (with correct units and to 3 significant figures):
345 K = ______°C
71.9 C
Use the following conversion factors to convert between the different temperature scales below.
Equations and calculations with values substituted:
Answer (with correct units and to 3 significant figures):
15.1 °F = ______ K
264 K
What are the 3 states of matter commonly found on earth?
Solid, Liquid, Gas
Describe a solid
fixed shape and volume
Describe a liquid
takes the shape of the container, has a fixed volume, forms horizontal surface
Describe a gas
takes shape and volume of container
Give a brief explanation of the law of conservation of matter.
The amount of matter involved in a chemical or physical change must remain constant.
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: coffee with cream and sugar
homogeneous mixture
appears visually the same throughout
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: nitrogen
element
pure substance that cannot be broken down
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: lemonade with ice
heterogeneous mixture
not uniform in composition
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: calcium chloride
compound
pure substance made from two or more elements
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: blood
homogeneous mixture
appears visually the same throughout
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: sodium fluoride
compound
pure substance made from two or more elements
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: chicken noodle soup
heterogeneous mixture
not uniform in composition
Classify the following types of matter as either an element, a compound, a homogeneous mixture, or a heterogeneous mixture and explain your reasoning: carbon
element
pure substance that cannot be broken down
Classify the following as either a physical or chemical property: The cylinder has a mass of 15 g.
physical
Classify the following as either a physical or chemical property: The ball is pink.
physical
Classify the following as either a physical or chemical property: Acetone is flammable.
chemical
Classify the following as either a physical or chemical property: The boiling point of water is 100°C.
physical
Classify the following as either a physical or chemical property: The paper is 30 cm long.
physical
Classify the following as either a physical or chemical property: Mercury is toxic.
chemical
Classify the following as either a physical or chemical property: The density of water is ~1 g/mL.
physical
Classify the following as either a physical or chemical property: Sulfuric acid is highly acidic.
chemical
Classify the following as either a physical or chemical change: An egg is fried.
chemical
Classify the following as either a physical or chemical change: A piece of paper is cut.
physical
Classify the following as either a physical or chemical change: A steak is cut into pieces.
physical
Classify the following as either a physical or chemical change: Food is digested in the stomach.
chemical
Classify the following as either a physical or chemical change: An ice cream cone melts.
physical
Classify the following as either a physical or chemical change: Sugar is dissolved in lemonade.
physical
Classify the following as either a physical or chemical change: Meat spoils.
chemical
Classify the following as either a physical or chemical change: Fireworks explode.
chemical
Classify the following as either an intensive or extensive property: The length of the table is 10 ft.
extensive
Classify the following as either an intensive or extensive property: The car is white.
intensive
Classify the following as either an intensive or extensive property: The coffee is at 70 °C.
intensive
Classify the following as either an intensive or extensive property: The volume of the solution is 50 L.
extensive
Classify the following as either an intensive or extensive property: The density of ethanol is 0.789 g/mL.
intensive
Classify the following as either an intensive or extensive property: Water boils at 100 °C.
intensive
Classify the following as either an intensive or extensive property: The book weighs 35 g.
extensive
For the scientists listed below, briefly summarize their major discoveries and the equipment and/or experiments they used in their discoveries: J.J. Thomson
J.J. Thompson used a cathode ray tube to calculate the mass-to-charge ratio of cathode ray particles and ultimately discovered the electron.
For the scientists listed below, briefly summarize their major discoveries and the equipment and/or experiments they used in their discoveries: Robert A. Millikan
Robert A. Millikan determined the charge of an electron using an “oil drop” experiment.
For the scientists listed below, briefly summarize their major discoveries and the equipment and/or experiments they used in their discoveries: Ernest Rutherford
Ernest Rutherford fired 𝛂-particles at gold foil which led to the discovery of the proton.
What are the 3 subatomic particles in a typical atom and their charges?
proton = +
electron = -
neutron = no charge
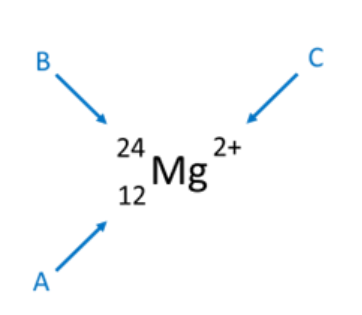
Label the parts (A, B, and C) of the chemical symbol below.
A. Atomic number
B. Mass number
C. Charge
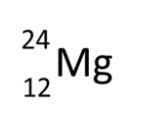
How would the following symbol be changed to show an isotope of Mg?
The mass number (24) would change but the atomic number (12) would stay the same.
Write the chemical symbols for the following: An element that has 17 protons, 18 electrons, and 18 neutrons.
3517Cl-1
Write the chemical symbols for the following: An element that has 24 protons, 18 electrons, and 28 neutrons.
5224Cr+6
Write the chemical symbols for the following: An element that has 34 protons, 36 electrons, and 45 neutrons.
7934Se-2
Fill in the missing information from the table below:
Element Symbol | 1. _________ |
Atomic Number | 47 |
Mass Number | 108 |
Protons | 2. _________ |
Electrons | 46 |
Neutrons | 3. _________ |
Atomic Charge | 4. _________ |
1. Ag
2. 47
3. 61
4. +1
Fill in the missing information from the table below:
Element Symbol | 1. _________ |
Atomic Number | 2. _________ |
Mass Number | 3. _________ |
Protons | 78 |
Electrons | 78 |
Neutrons | 117 |
Atomic Charge | 4. _________ |
1. Pt
2. 78
3. 195
4. 0
Fill in the missing information from the table below:
Element Symbol | F |
Atomic Number | 1. _________ |
Mass Number | 19 |
Protons | 2. _________ |
Electrons | 3. _________ |
Neutrons | 4. _________ |
Atomic Charge | 1- |
1. 9
2. 9
3. 10
4. 10
A sample of sulfur is found to contain 94.93% of 32S atoms (mass 31.972 amu), 0.76% of 33S atoms (mass 32.971 amu), 4.29% of 34S atoms (mass 33.968 amu) and 0.02% of 36S atoms (mass 35.967 amu) . Calculate the average mass of a S atom.
Setup:
Answer (with correct units and to 4 significant figures):
Average Mass of Sulfur = (0.9493 x 31.972amu) + (0.0076 x 32.971amu) + (0.0429 x 33.968amu) + (0.0002 x 35.967amu)
Answer: 32.07 amu