UCLA Chem 20B Felker Winter 2018
1/214
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
215 Terms
Gas
Anything that expands to occupy the space of its container
Ideal Gas Law
PV=nRT
Pressure x Volume = Number of Moles x Ideal Gas Constant x Temperature
Pressure
Caused by change in momentum that is caused by gas molecules bouncing off container walls
Force/Area
Hydrostatic Pressure
ρgh = Density (Rho, not "p") x Gravity x Height
Units for Pressure
Force/Length^2 or Energy/Volume
Pascals, Bars, Atmospheres, Torrs
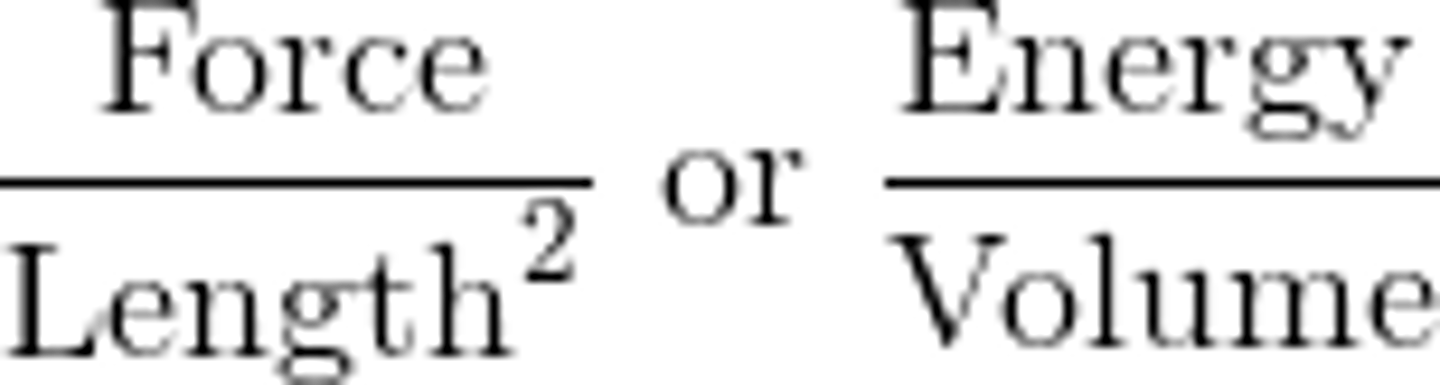
Temperature
Proportionate to Kinetic Energy
Thermal Contact
state of two or more objects or substances in contact such that heat can flow from one to the other
0th Law of Thermal Dynamics
If object A is in thermal equilibrium with object B and object B is in thermal equilibrium with object C, then Object A is also in thermal equilibrium with object C
Triple Point Temperature
A temperature where something's (water's) solid, liquid, and gaseous states are all present
Units of Temperature
Kelvin, Celsius, Fahrenheight
Converting from Celsius to Kelvin
C = K - 273.15
Point Mass
Random Velocities, no interaction
How is an ideal gas different from a real gas?
Real gasses interact at a distance
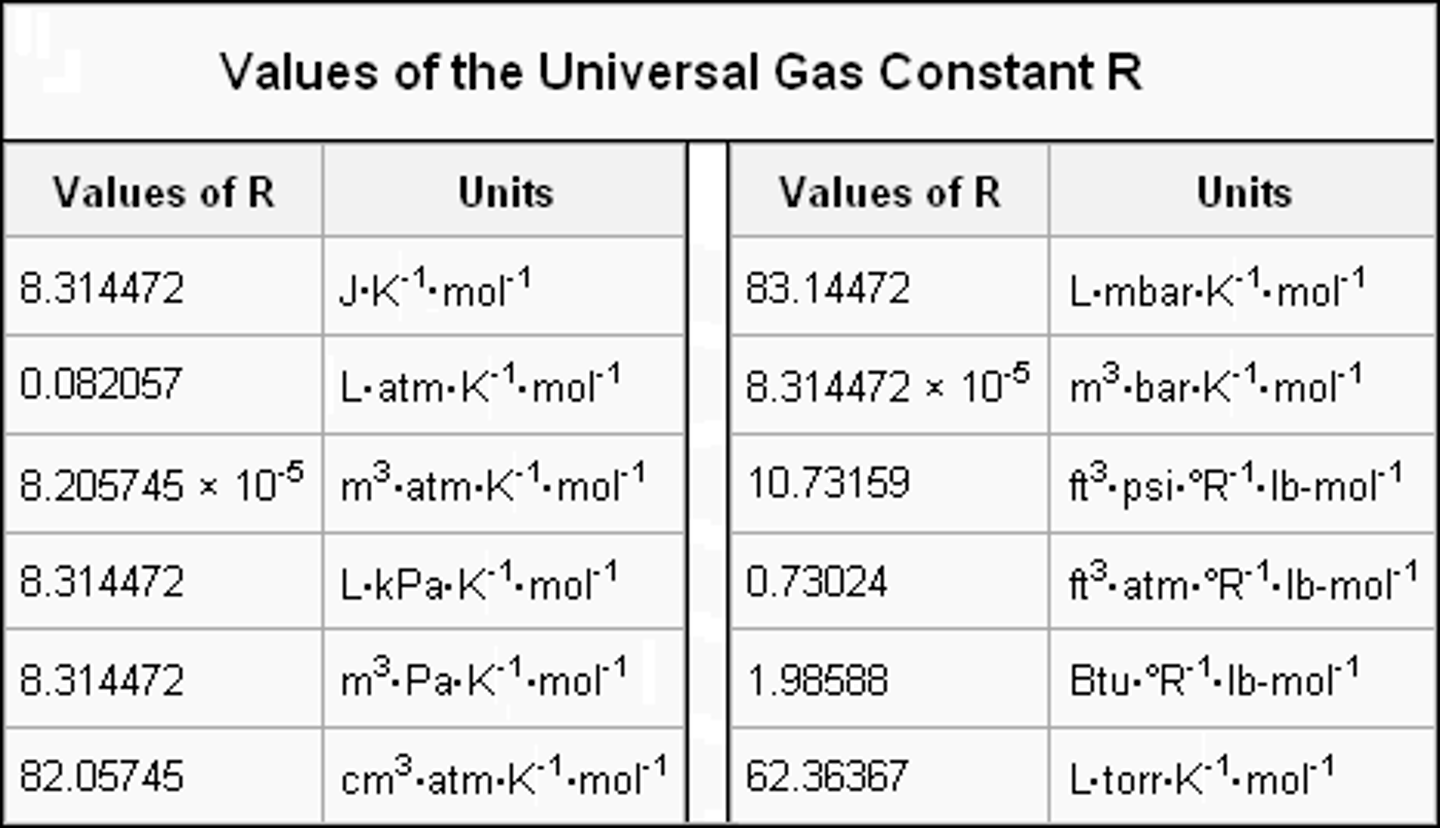
Ideal gas constant
R ; in energy per (mol*temperature) or (pressure x volume)/(mol x temp)
8.314 j/mol x k
8.134 x 10^-2 bar x L/mol x k
8.21 x 10^-2 atm x L/ mol x k
Boyle's Law
P_1 x V_1 = P_2 x V_2
Pressure and volume are inversely proportional (when number of moles and temperature remain constant)
Pressure is proportionate to the #of collisions per time per are...so a smaller volume means there will be more collisions and a higher pressure
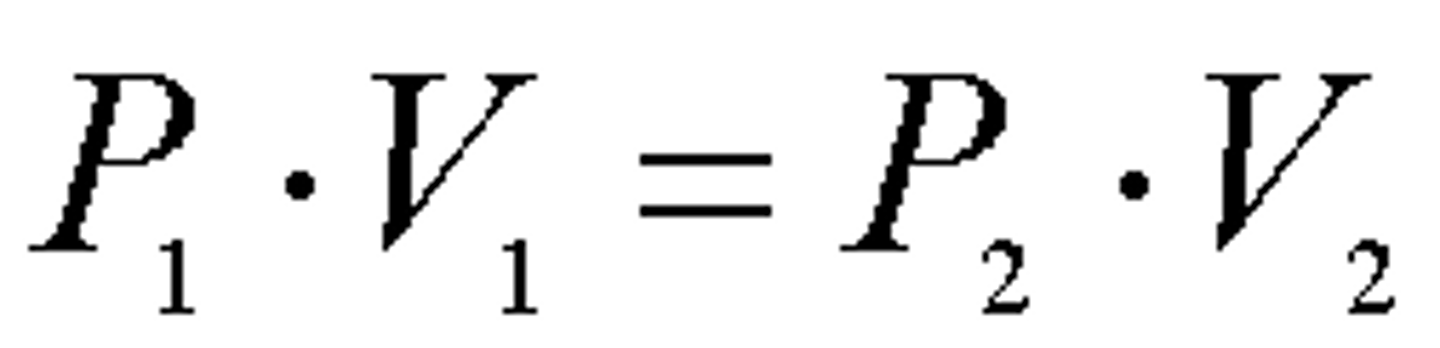
Charles Law
Volume is proportionate to temperature (when number of moles and pressure are constant)
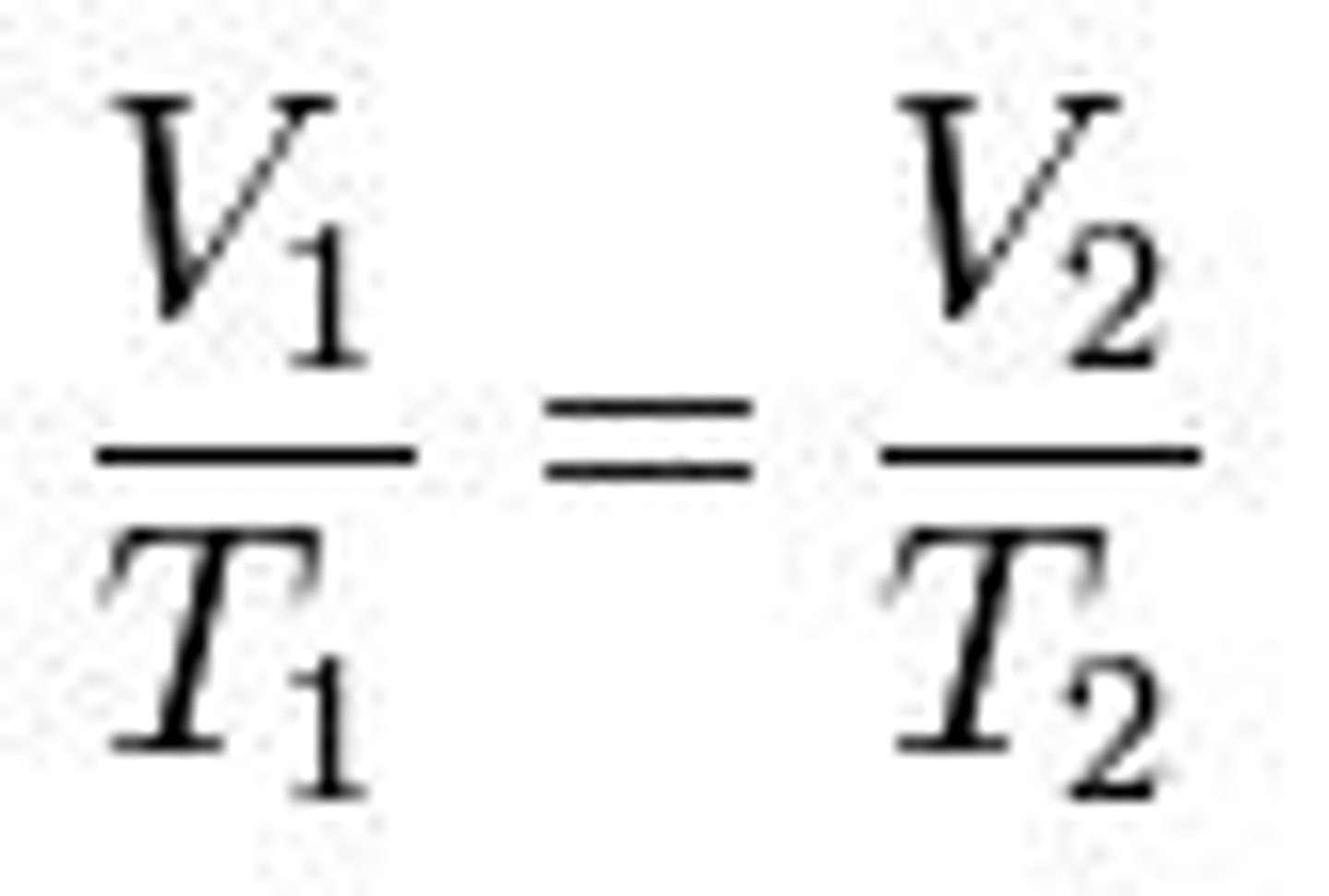
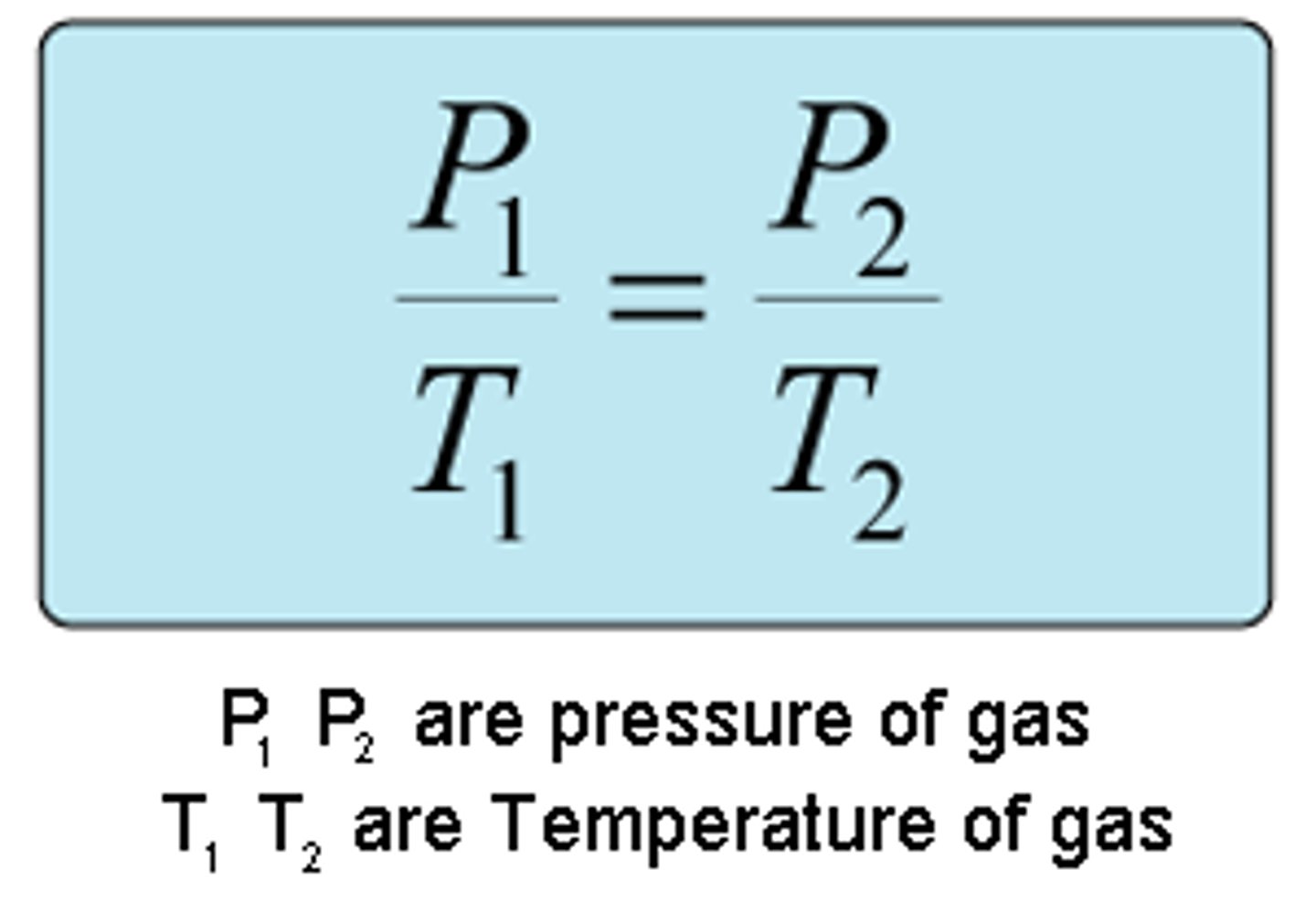
Guy-Lussac's Law
Pressure is proportionate to temperature (when number of moles and volume are constant)
STP
Standard Temperature and Pressure (0 degrees celsius and 1 ATM)
Avogadro's Law
Volume is proportionate to number of moles (when temperature and pressure are constant)
Ideal volume
RT/P
24.790 Liter/mol (at STP)
SATP
Standard ambient temperature and pressure
25 degrees celsius and 1 bar
Mole Fraction
x sub i ; number of moles sub i / number of moles total
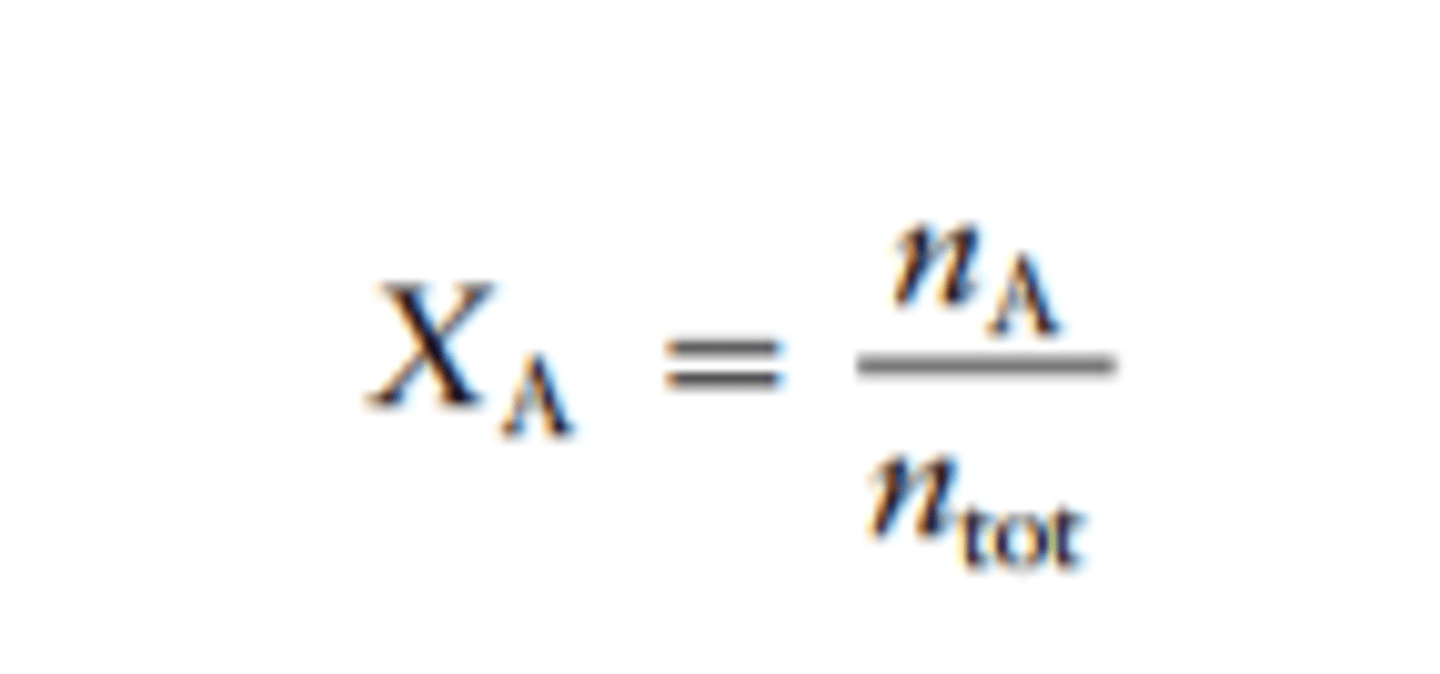
Dalton's Law
P sub i = (n sub i * RT)/V
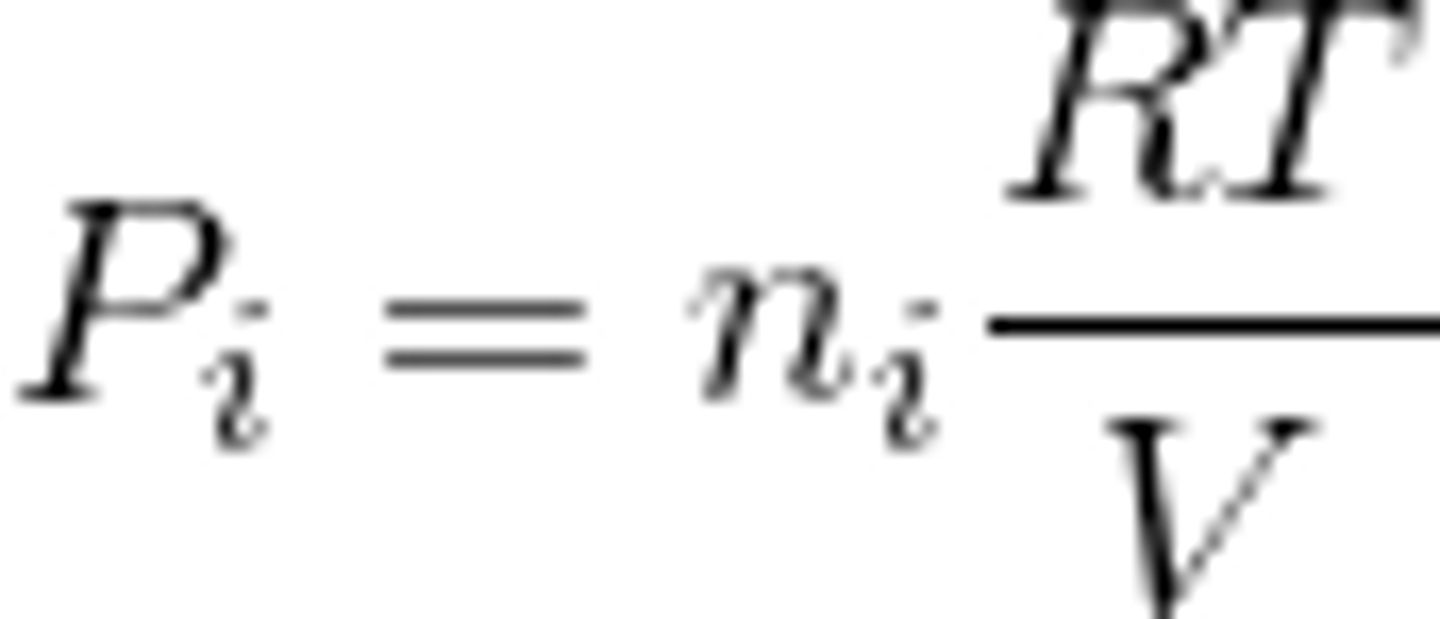
Kinetic Theory of Gasses
"gases are made up of tiny particles in random, straight line motion. They move rapidly and continuously and make collisions with each other and the walls."
Boltzmann's Constant
R/Avogadro's Number
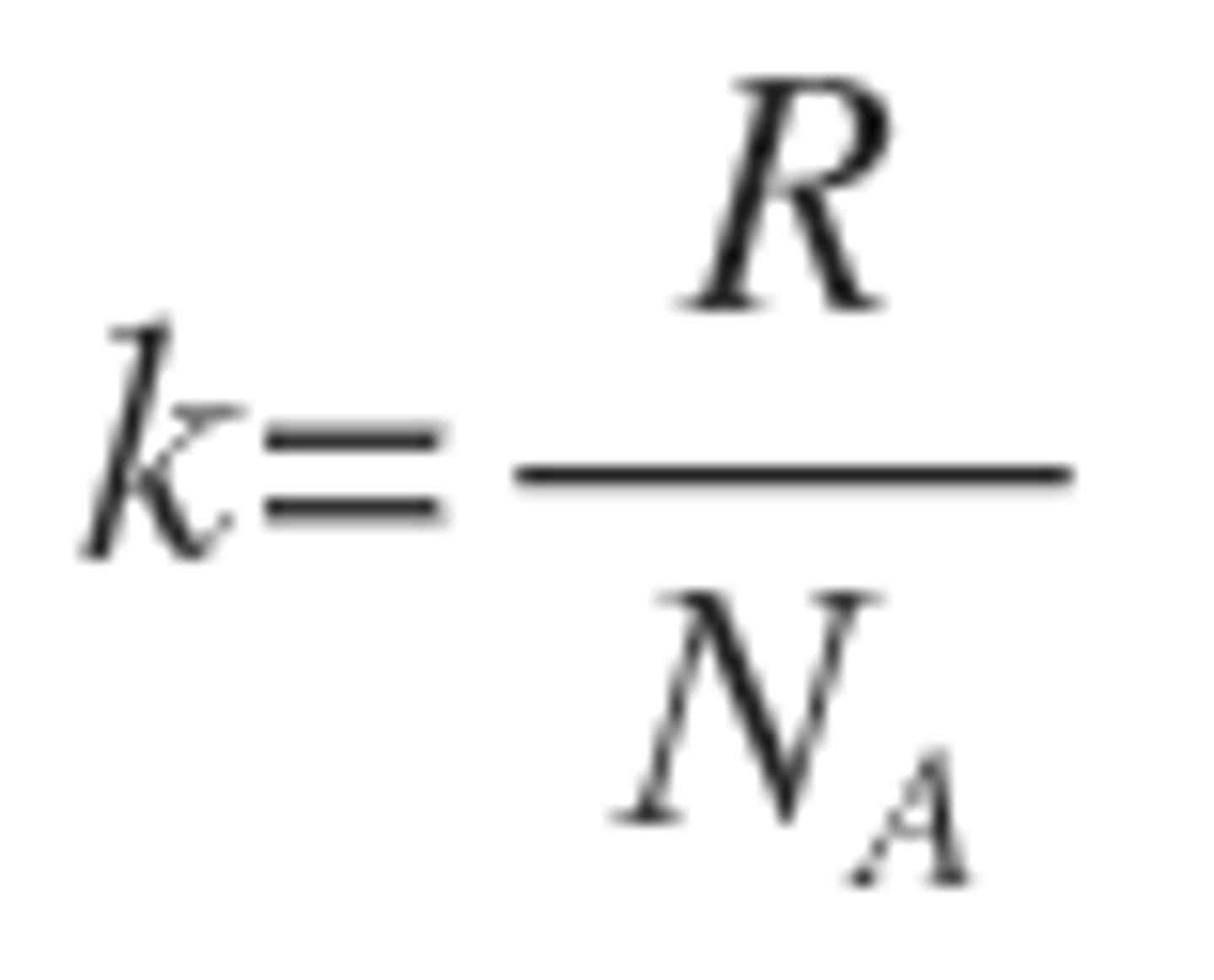
Root Mean Speed
V sub(rms) = sqrt(3RT/M)
or Square root of 3 *(Ideal Gas Constant x Temperature in Kelvin )/Molar Mass
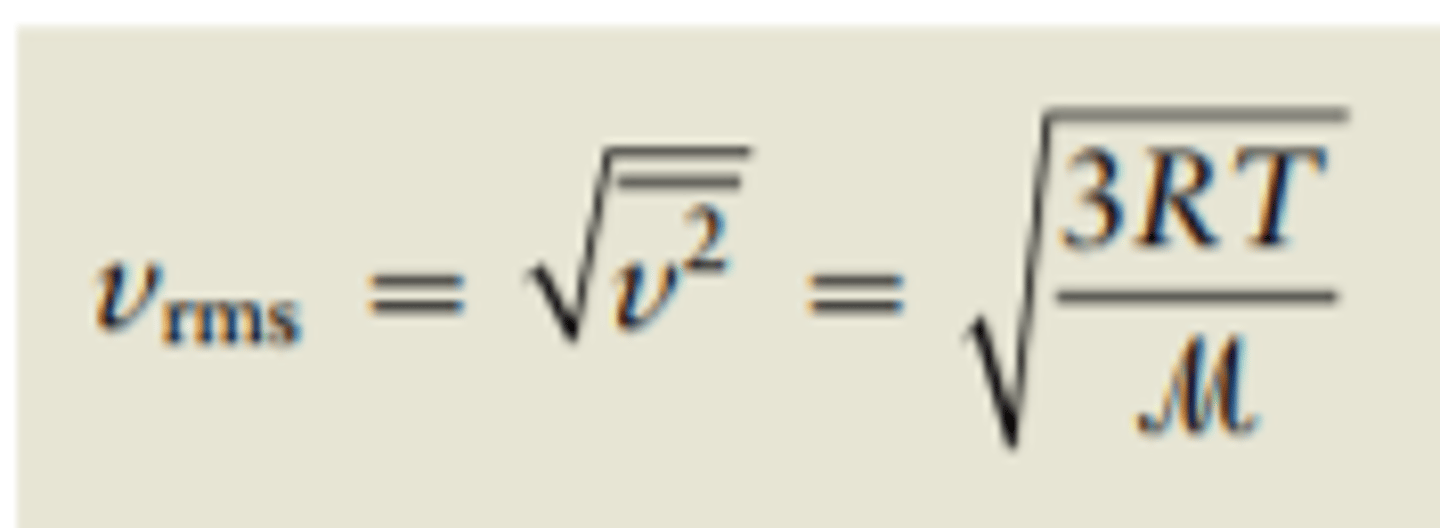
Probability that a particle has speeds between v1 and v2
F = probability particle has some speed between v and v + dv
integral from v1 to v2 of f(v)dv
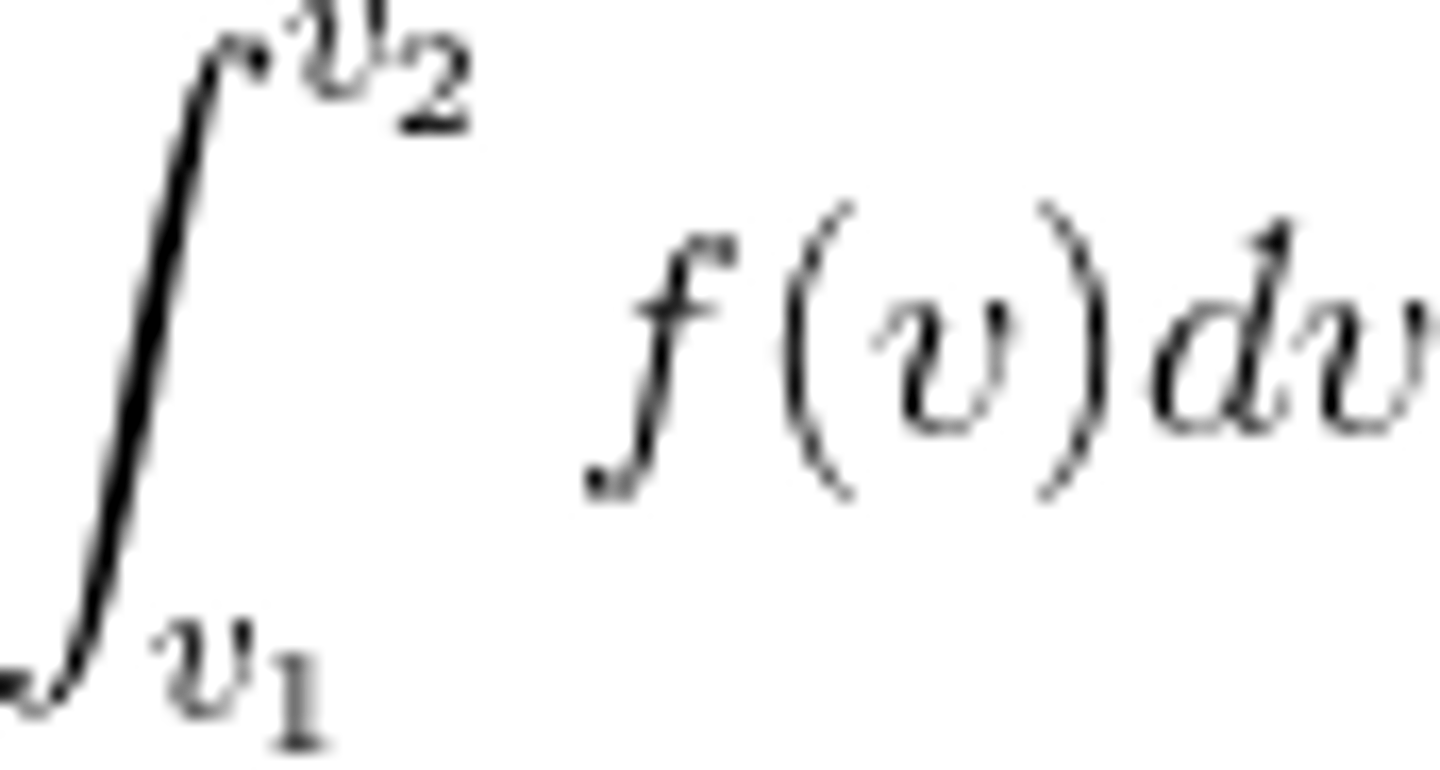
Maxwell-Boltzmann Speed Distribution
Sort of like a bell shaped curve
f(v) = 1/(pi/2 x KbT/M)^(3/2) x v^2((-mv^2)/2KbT)
Note that the first term is normalization
Boltzmann's Factor
-KE/KbT
negative kinetic energy over Boltzmann's constant times temperature
What happens to Maxwell-Boltzmann speed distribution as temperature increases
Max speed increases, peak (most probable speed) is lowered (fewer species) but faster (shifted rightwards)
This is equivalent to having mass decrease
Most Probable Speed from Maxwell-Boltzmann Speed Distribution
At the peak, or critical point.
Where f'(v) = 0
sqrt(2KbT/M)
Square root of 2 x Boltmann's Constant x Temperature / Molar Mass
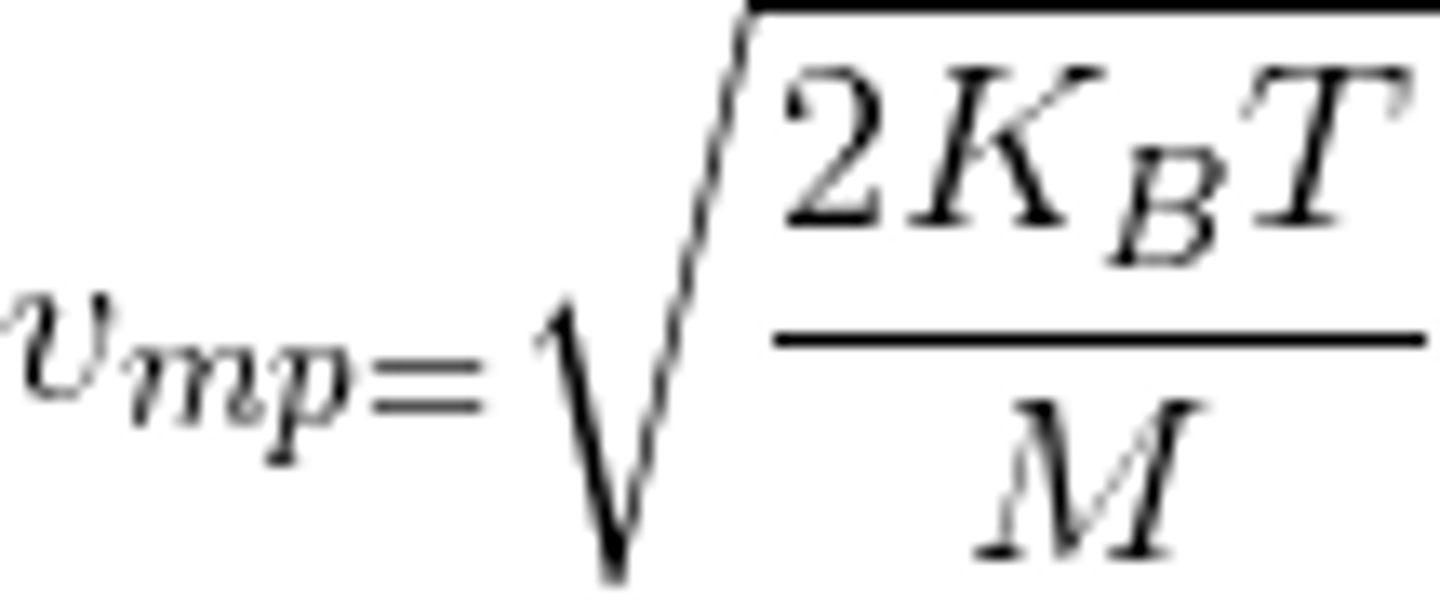
Average speed of Maxwell-Boltzmann distribution
v-bar = sqrt(8KbT/Pi x m) or sqrt(8RT/pi x M)
Square root of 8 times Boltzmann's constant x temperature over pi x mass
or
square root of 8 times ideal gas constant times temperature over pi time molar mass
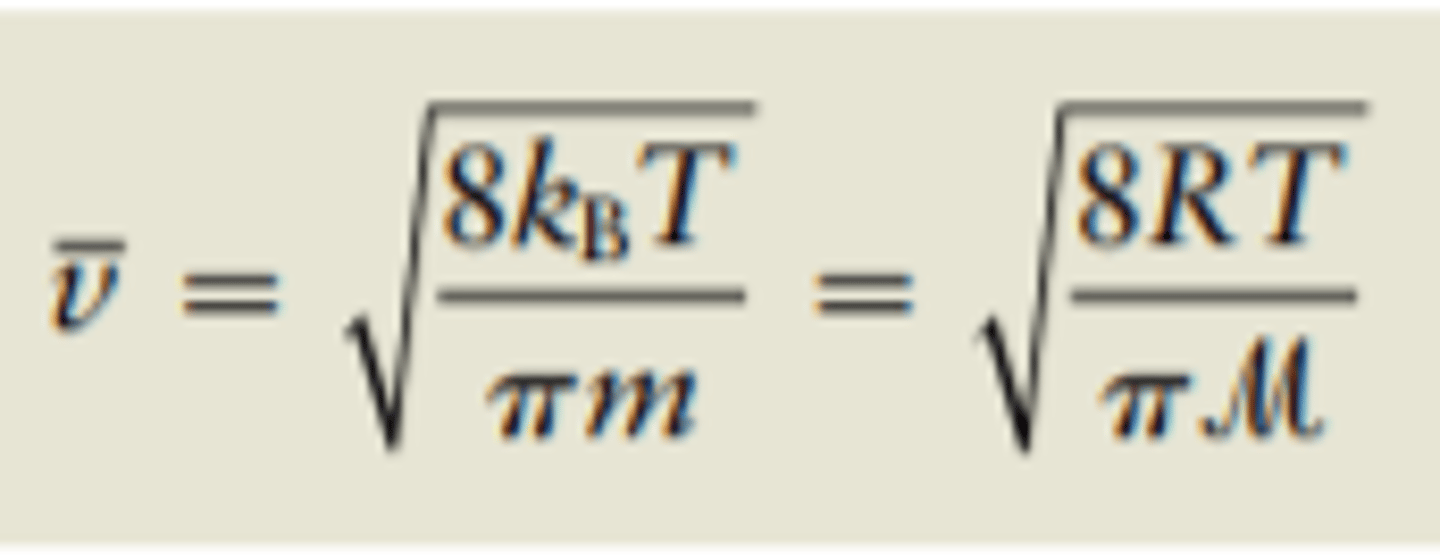
Ratio of most probable speed to average speed to root mean square speed
Vmp:V-bar:Vrms =
1 : 1.128 : 1.225
Effusion
df/dv
Rate gas will leave a hole (small hole like a pin hole)
The ratio of the numbers of molecules of the two species effusing
through the hole in a short time interval.
N = Number of Molecules.
M = Molar Mass
B is the heavier molecule (heavier than A)

Diffusion
How quickly a contaminant spreads when introduced
When is PV= nRT not true for real gasses?
When pressure increases
More pressure = more interactions
Energy-Distance Graph
Graph of V(r) where r = distance
Has repulsive wall, where it takes a lot of energy to make molecules get close together due to coulombic forces
Potential Well, where things are stable
Attractive Region; to the right of the potential well
Compression Factor
For ideal gasses, it equals one.
When z is not equal to one, the ideal gas law doesn't work
At intermediate pressures, attractive forces come into play, and z is less than 1 (collision speeds decrease = pressure decreases)
At high pressures, distance decreased and repulsive forces come into play; z is more than 1. (collision speeds increase = pressure increase)
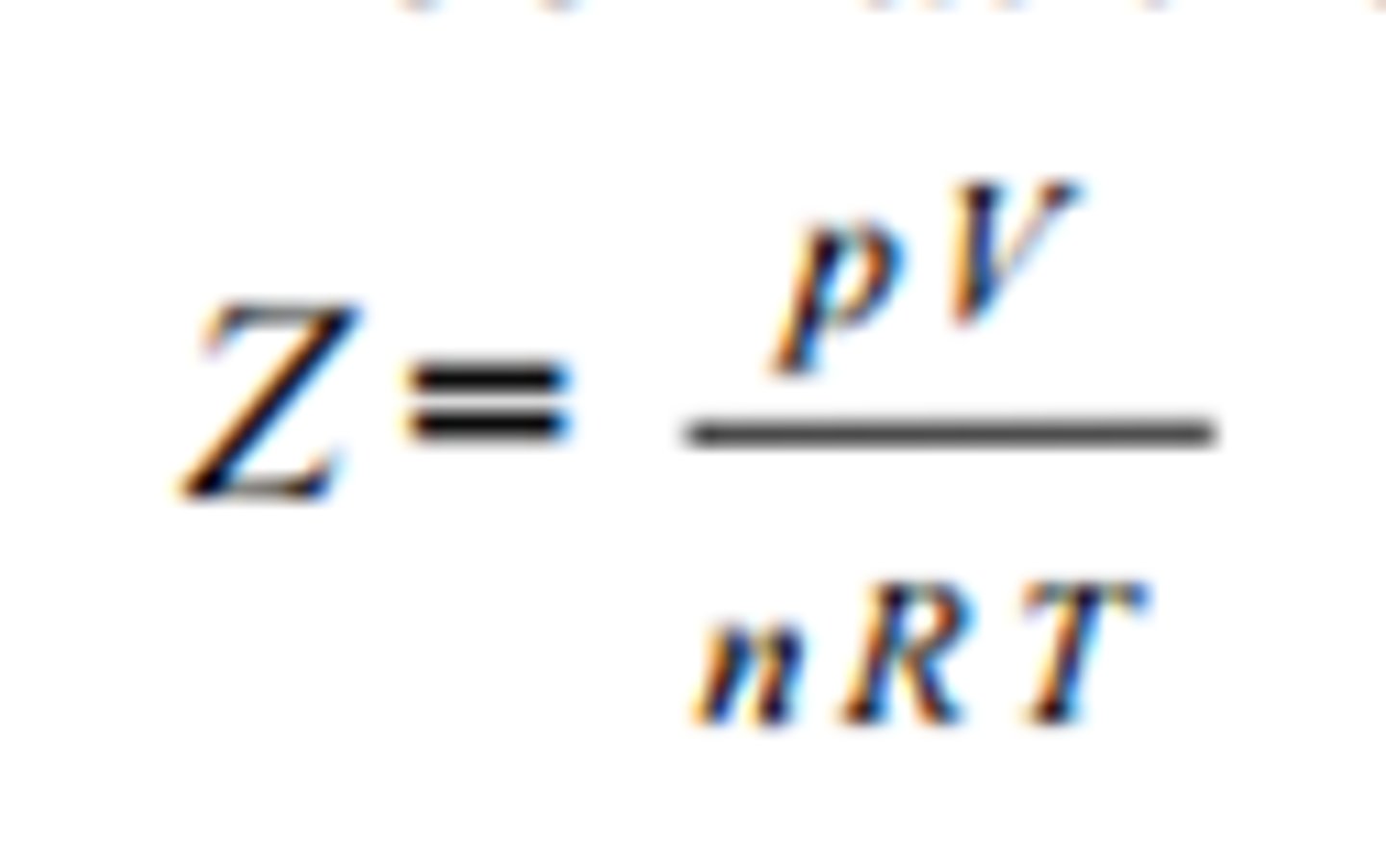
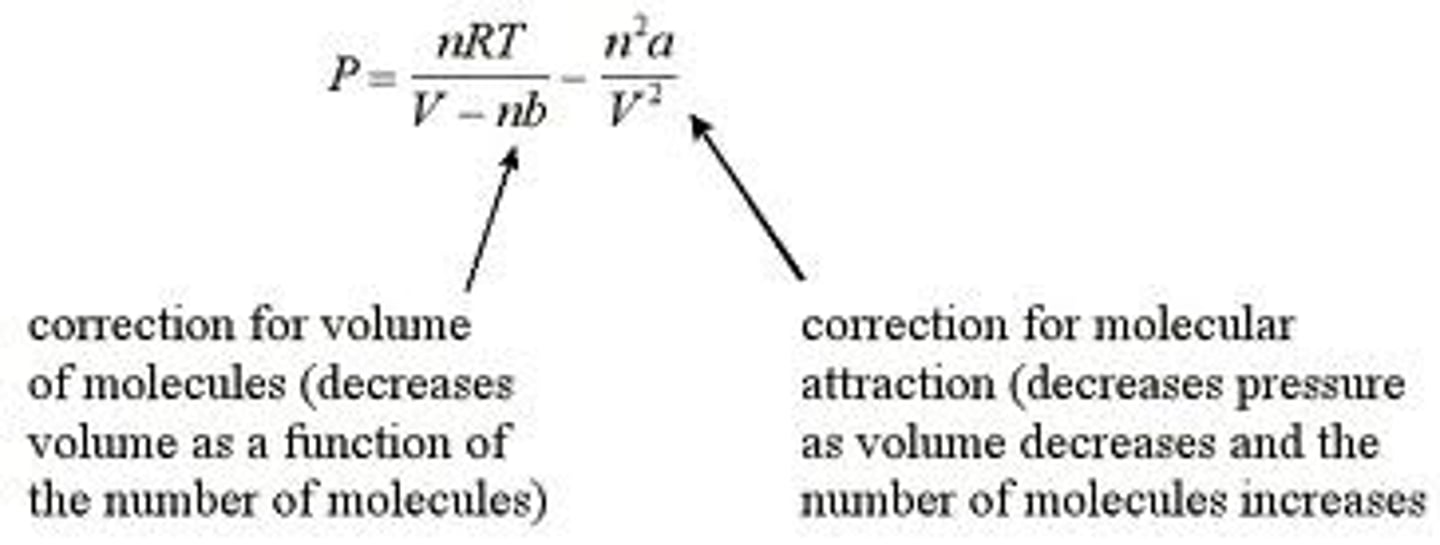
Van der Waal's equation
An upgrade of the ideal gas law
V-nb is accessible volume, the total volume subtracted by the occupied space. As b increases, there is less space, more repulsion, and more pressure
a helps increase the attractive force and decrease the pressure
Volume of Typical Gas
25 Liters per Mol
To find volume when given density and molar mass
Molar Volume = Density/Molar Mass
Typical distance between gas molecules
34 angstroms
Typical distance between liquid molecules
3 angstroms
Distance between neighbors for gas, liquid, and solid
Most distance in gas, appreciable distance in a liquid, and little distance in solid
Compression
Fractional decrease in volume
per unit increase in pressure. Temperature is constant
k = kappa
k = [delta(v)/delta(k)]_t x (-1/V)
in an ideal gas it is 1/P
in a solid it is hard to compress
![<p>Fractional decrease in volume</p><p>per unit increase in pressure. Temperature is constant</p><p>k = kappa</p><p>k = [delta(v)/delta(k)]_t x (-1/V)</p><p>in an ideal gas it is 1/P</p><p>in a solid it is hard to compress</p>](https://knowt-user-attachments.s3.amazonaws.com/947486ee-e56a-4f18-b96c-7de906981d06.png)
Thermal Expansion
Fractional increase in the
volume of a substance per degree increase in temperature. Pressure is constant
alpha = [delta(v)/delta(t)]_p x (1/V)
In an ideal gas it is 1/t
![<p>Fractional increase in the</p><p>volume of a substance per degree increase in temperature. Pressure is constant</p><p>alpha = [delta(v)/delta(t)]_p x (1/V)</p><p>In an ideal gas it is 1/t</p>](https://knowt-user-attachments.s3.amazonaws.com/be47f327-e477-413b-b4d3-f9f1cb9c1c71.png)
Fluidity
The ability of a substance to flow...opposite of rigidity
Rigidity
The physical property of being stiff and resisting bending...opposite of fluidity
Diffusion
Movement of molecules from an area of higher concentration to an area of lower concentration.
Easier in gasses than in liquids. Easier in liquids than in solids.
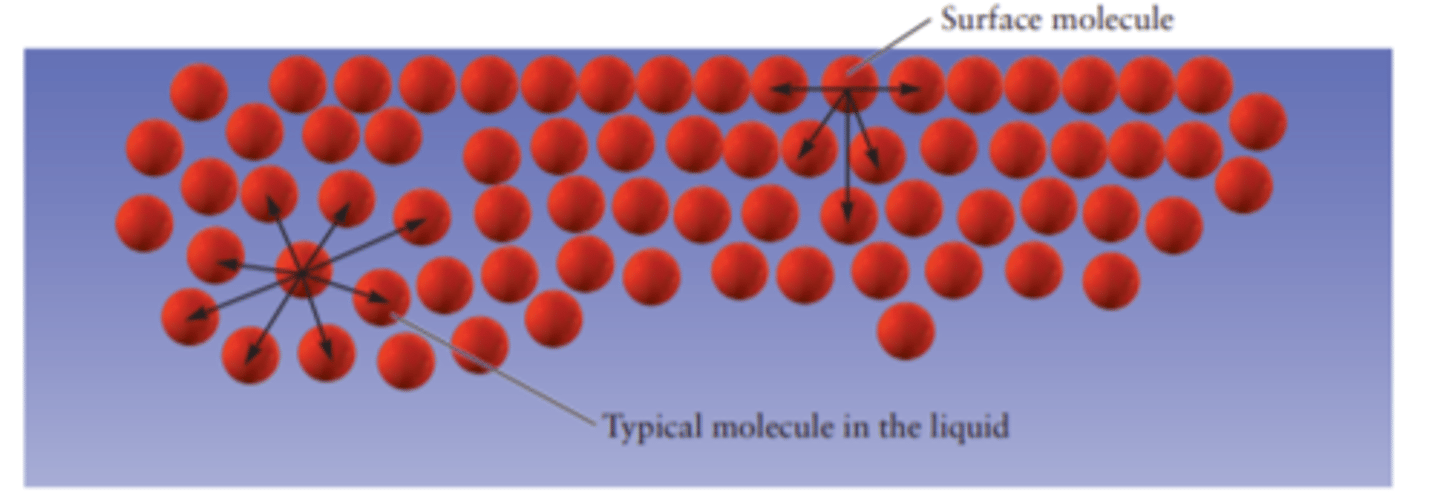
Surface Tension
Amount of energy required to increase surface area.
To increase surface area, bulk molecules (not in surface) need to go to surface, meaning that bonds will break
Inter vs Intra Molecular forces
Inter = different molecules
Intra = between nuclei within a molecule
Intermolecular forces are weaker and longer range than intramolecular forces. Direction in intermolecular forces are less relevant
Ion-Ion Force
Strong, non-directional, coulumbic interaction.
Happens in both intra and inter molecular contexts
Scales to 1/R (very long range)
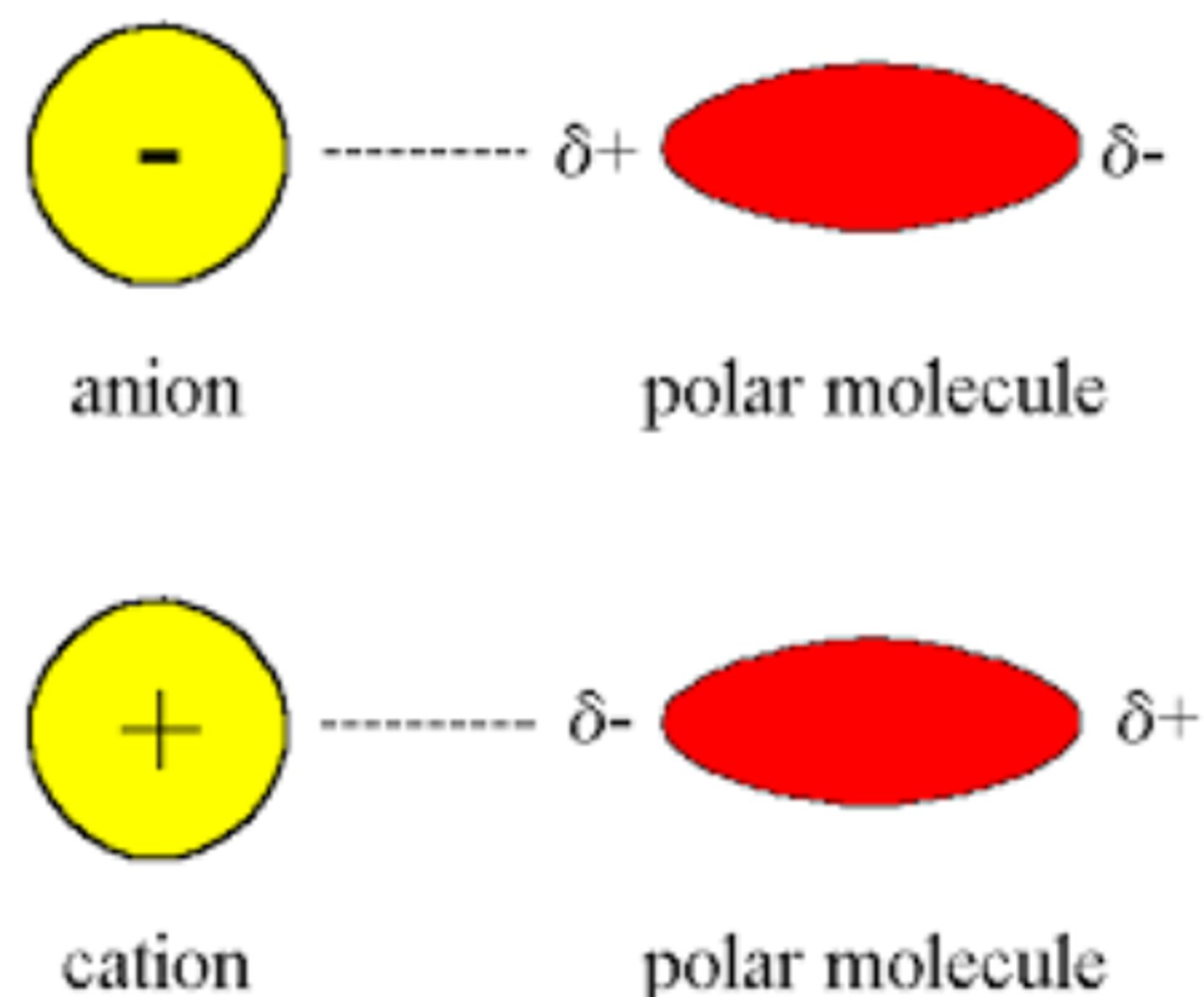
Dipole-Ion Force
A neutral molecule (permanent dipole) interacts with a charged molecule.
Strong and directional interaction
Range is 1/R^2 (long range, falls off more rapidly than ion-ion)
Dipole-Dipole Force
Dominant force between polar molecules.
In a dipole, there is a negative and positive part. Sometimes they align and stuff.
Weak, directional, almost always attractive, and short range interactions
Range is 1/R^3
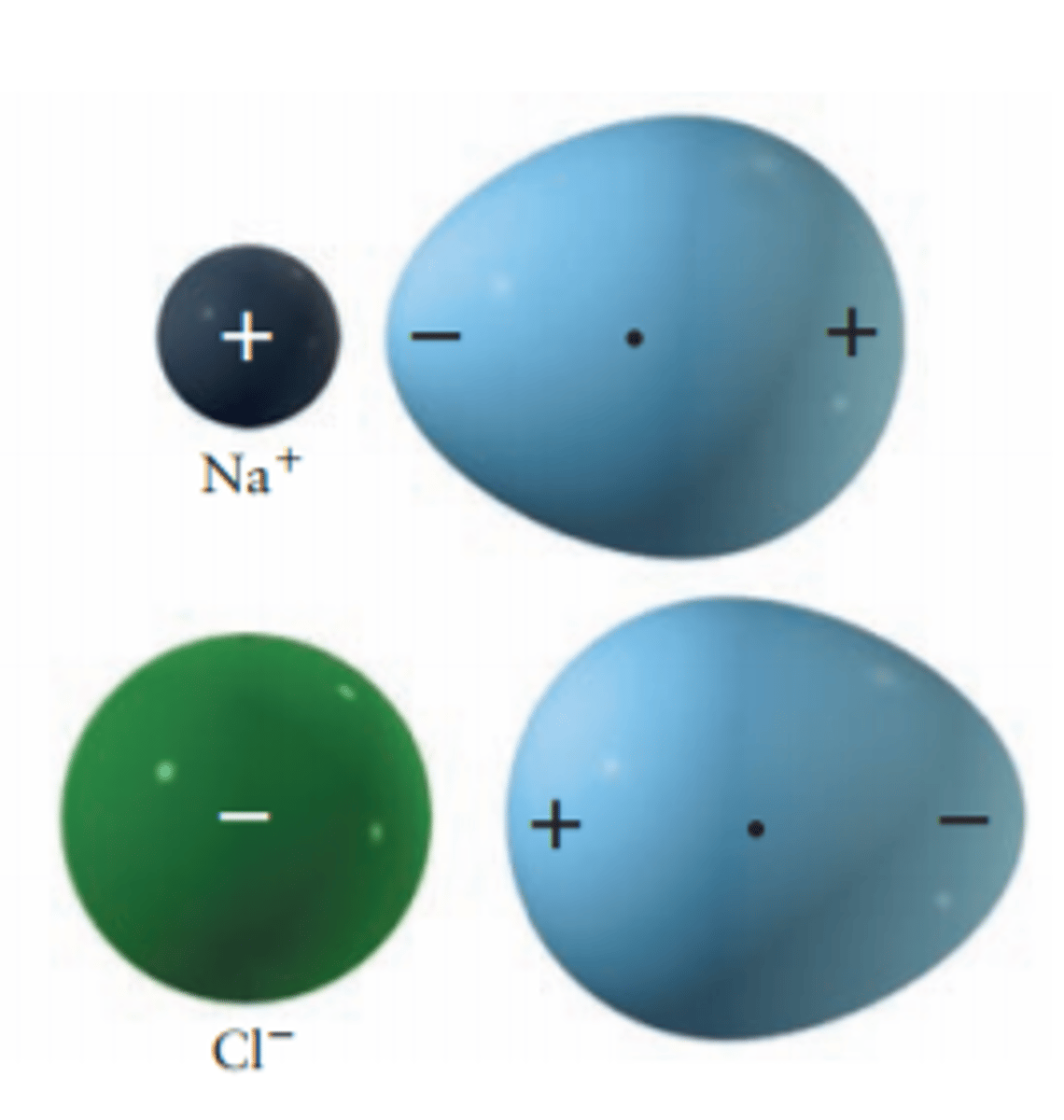
Induced Dipole Force
Approaching charged atoms/molecules can induce a dipole upon a neutral atom
In the pic the blue thing is an Argon
Range is 1/R^4
Weak, directional, attractive, and short range
Polarizability
Electrons respond to electric field.
Scales with number of electrons (more electrons = more polarizeable)
Applies to both atoms and molecules
Dipole Induced Dipole Force
A dipole induces another dipole.
A dipole (like HF) induces a dipole upon a neutral atom.
Range is 1/R^5
Weak, directional, attractive, and short range
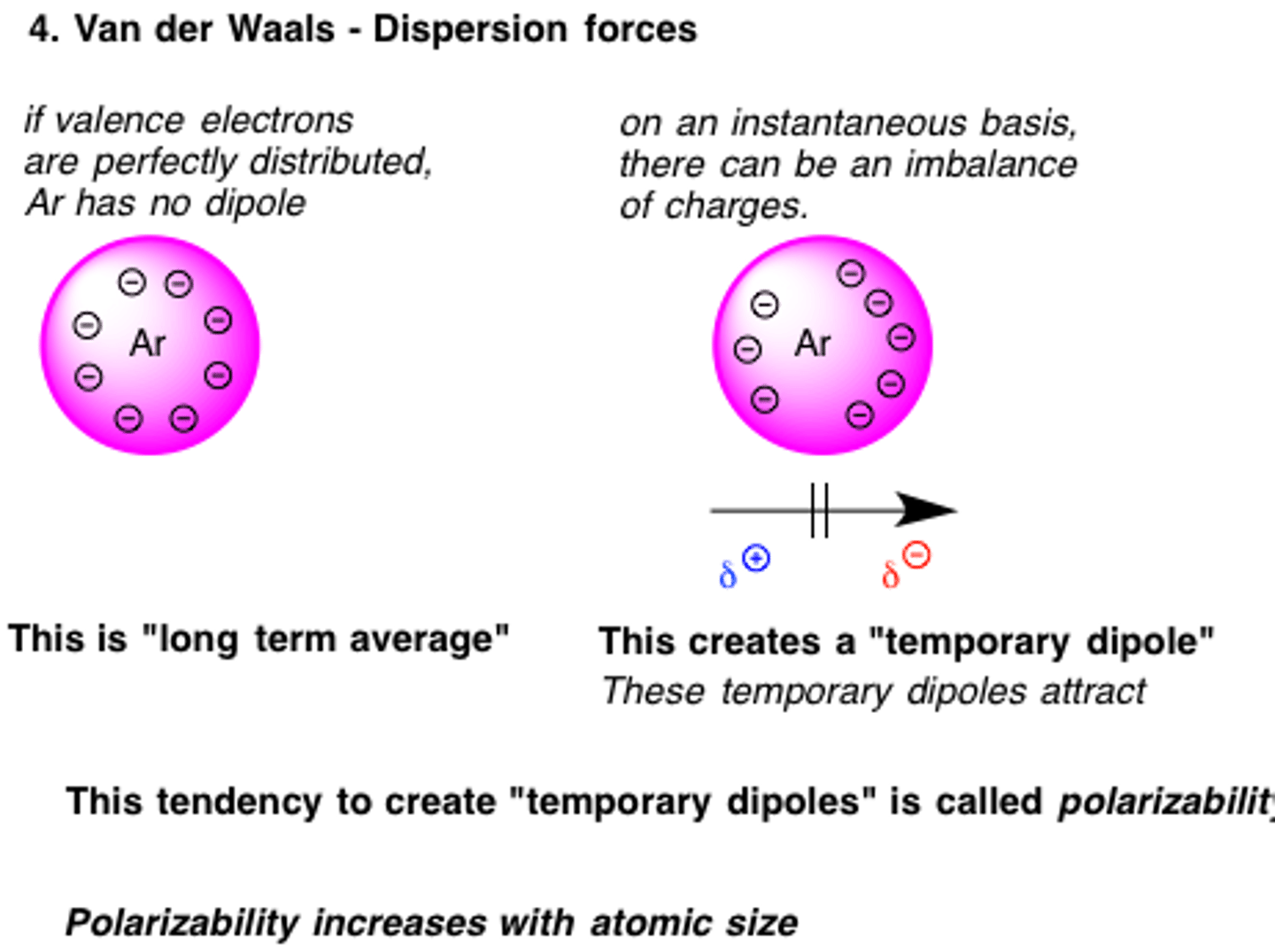
London Dispersion Forces (aka van der Waals forces)
Happens momentarily/spontaneously and is very weak. Has a very short range and is directional
A temporary dipole is created when there is a mismatch of electrons
Should be able to happen to anything with electrons, even noble gasses
Range is 1/R^6
Exchange Repulsive Force
Short range and always repulsive.
Originates from Pauli Exclusion principle.
When electrons get too close...they are not supposed to have the same position/spin and stuff
Range is 1/R^16
Boiling
When a liquid escapes to the gas phase.
It needs more energy to break bonds
More attractive interactions/forces = higher boiling point aka harder to boil
Order of intermolecular forces
Ionic > Hydrogen bonding > dipole dipole > Van der Waals dispersion forces
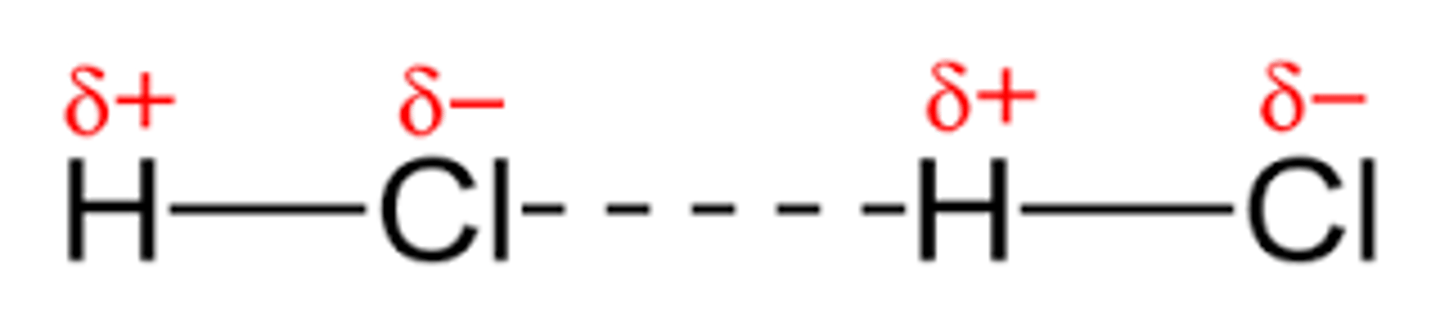
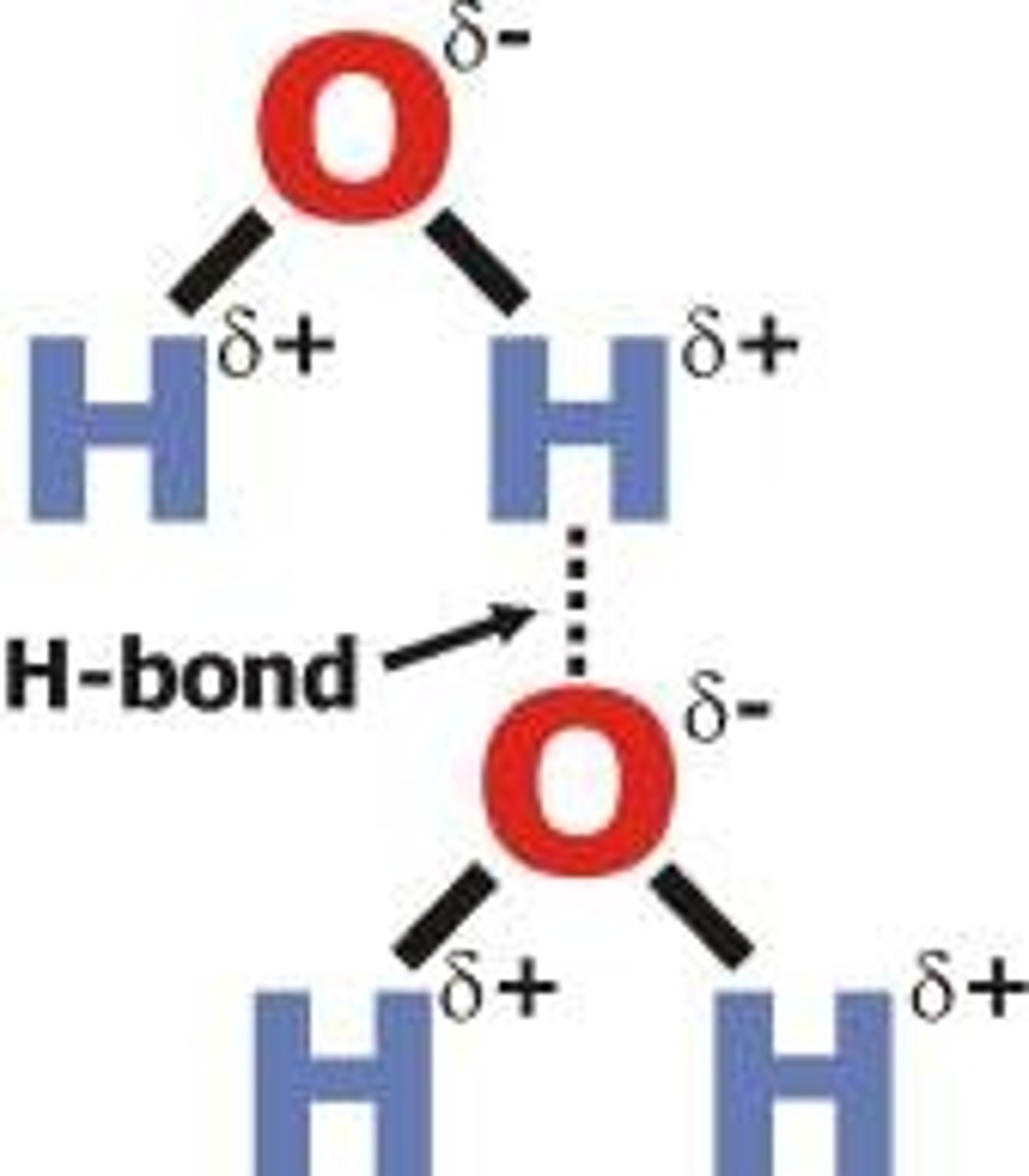
Hydrogen Bond
A dipole-dipole force on steroids, when hydrogen bonds with an extremely electronegative element (F, N, O)
Very strong...
Positive part of a dipole gets close and comfy with a negative part
Phase
One of the three forms--solid, liquid, or gas--which every substance is capable of attaining;
A sample of matter that is uniform throughout, both in its chemical constitution and in its physical state
Vapor Pressure
(P_vap) The pressure of the vapor coexisting with
a confined liquid or solid at any specified temperature.
This is a function of temperature
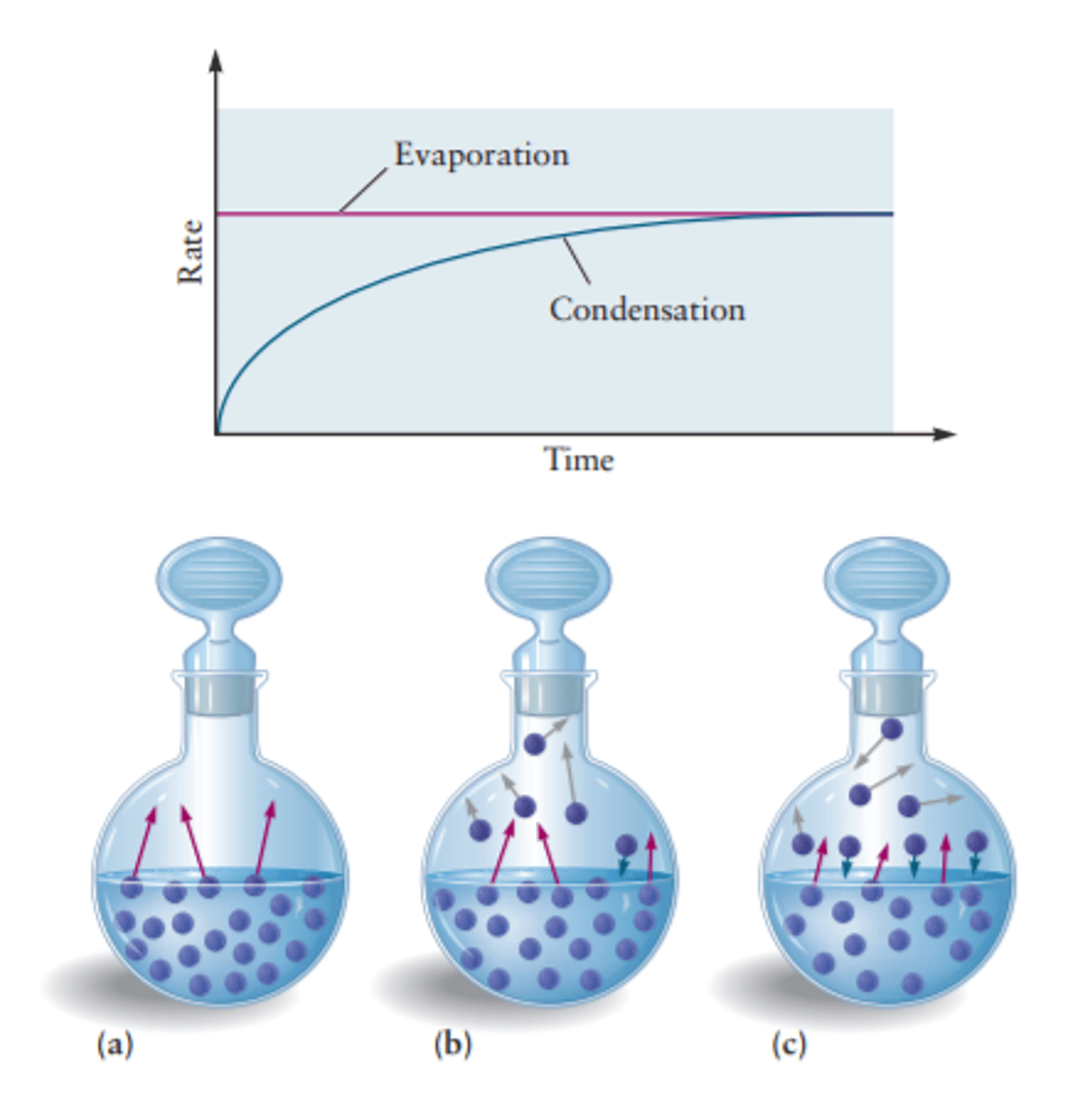
Achieving Phase Equilibrium (Liquid and Gas)
If we add a liquid into an empty flask (vacuum), eventually both a liquid and a gas will be present. This is because liquid some molecules at the surface will have enough energy to break off, or evaporate.
Once there are gasses in the system, some of them will crash into the surface of the liquid and lose its energy, or condense.
Equilibrium is reached when the rate of evaporation is equal to the rate of condensation
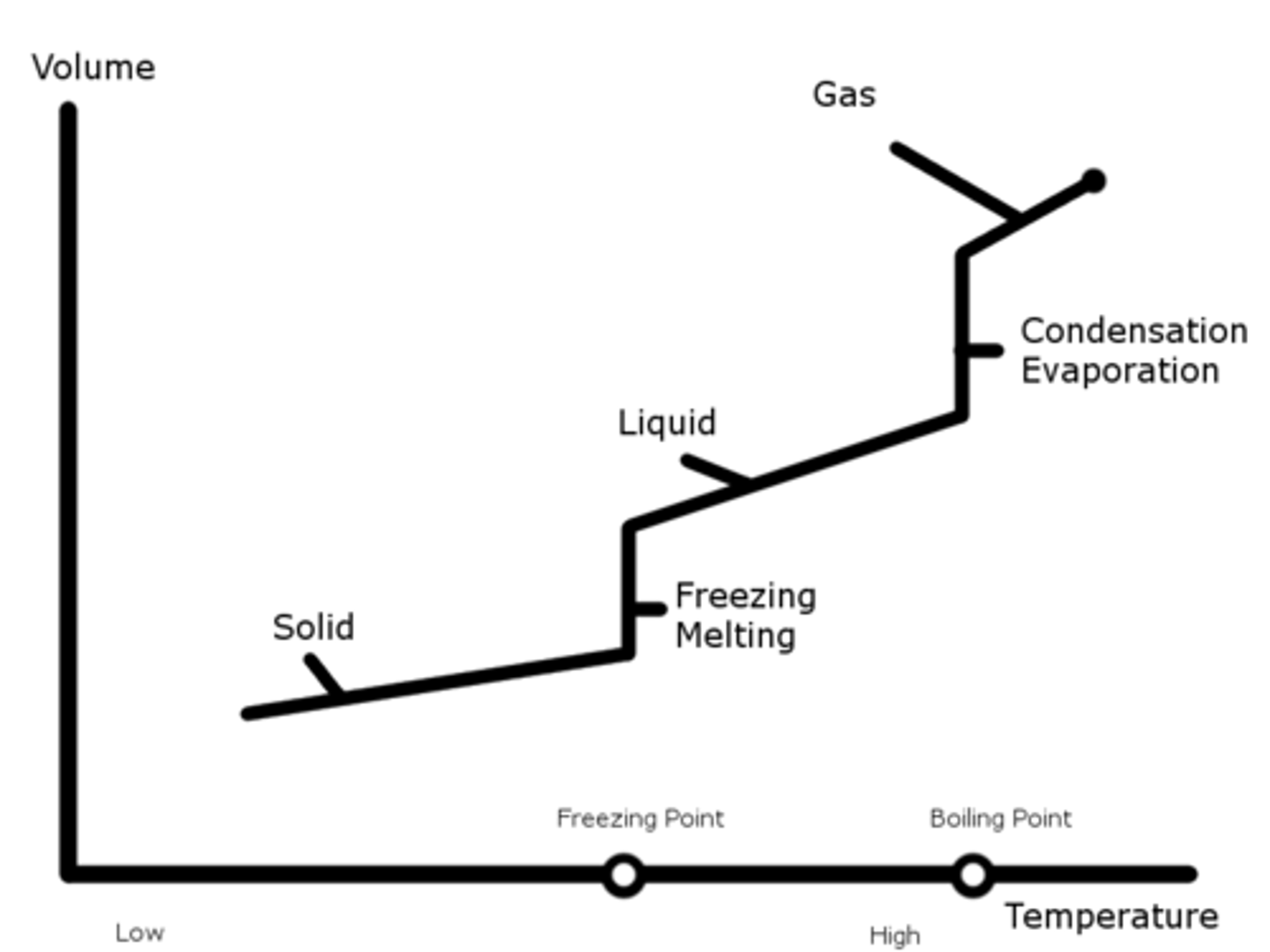
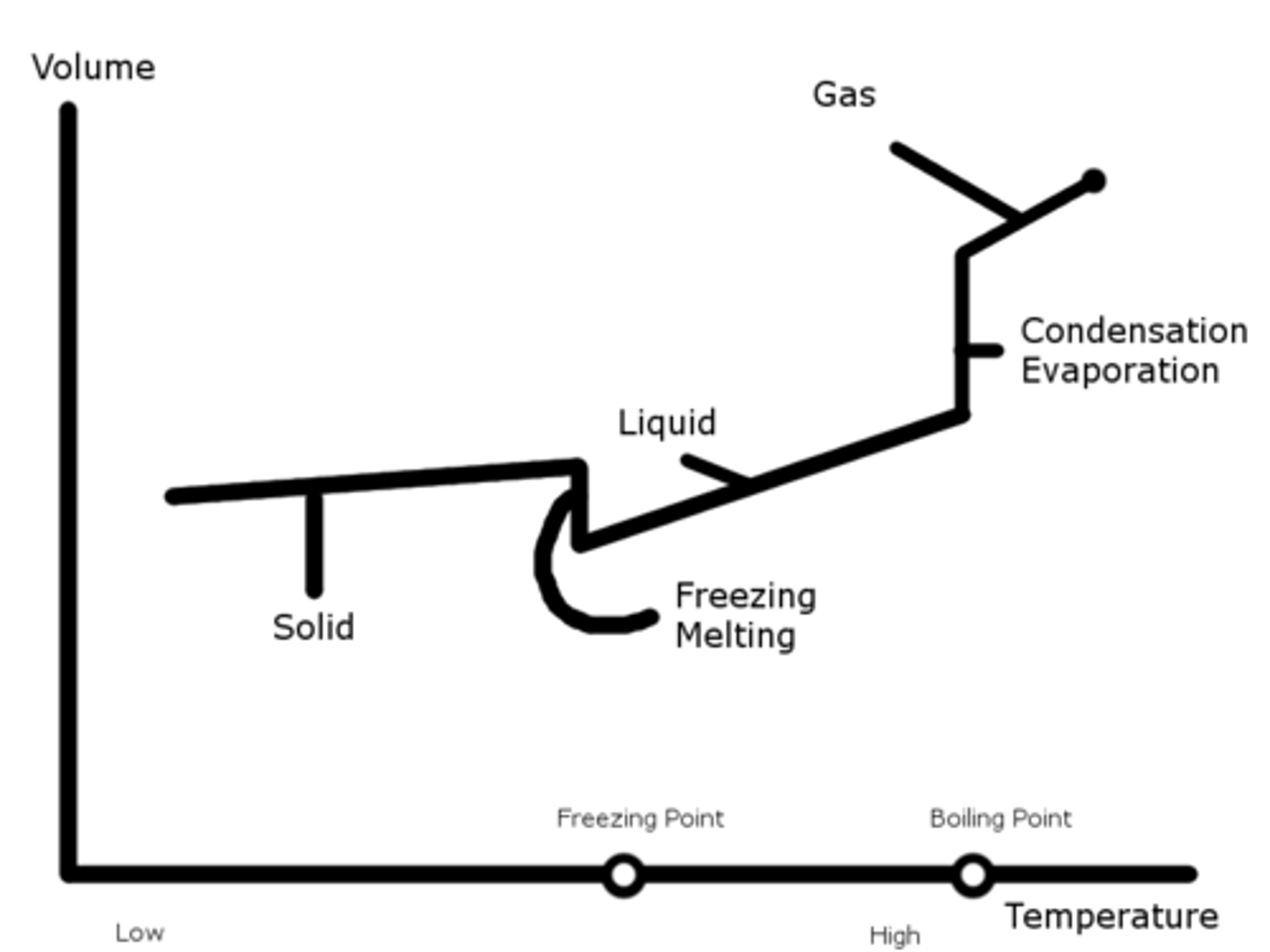
Phase Change (Volume-Temperature Chart @ Constant Pressure)
As temperature decreases, molecules go from a gaseous to a liquid, and to a solid phase.
During the phase changes, the volume will change but the temperature will change. At this time, the two phases will be in equilibrium and coexist.
Volume - Temperature Phase Change Chart for Water
Water expands as it freezes, so after transitioning from water to ice, the volume will increase
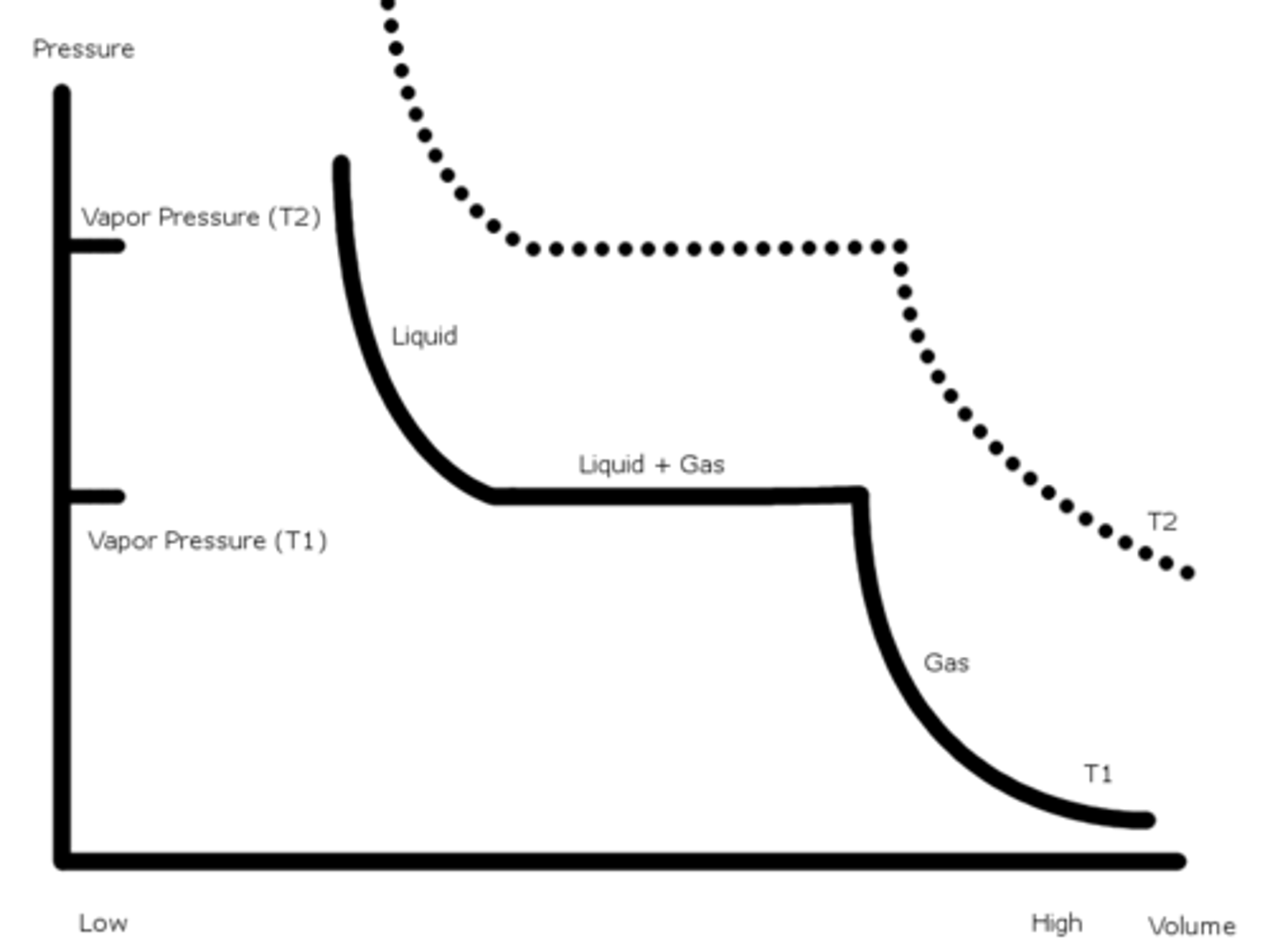
Pressure-Volume Phase Change graph (@constant temperature)
We are squeezing a sample and watching as pressure builds up.
We can observe that the flat lines are where the gas and liquid phases are in equilibrium. This is associated with the Vapor Pressure at the temperature.
If we had a higher constant temperature, the vapor pressure will increase
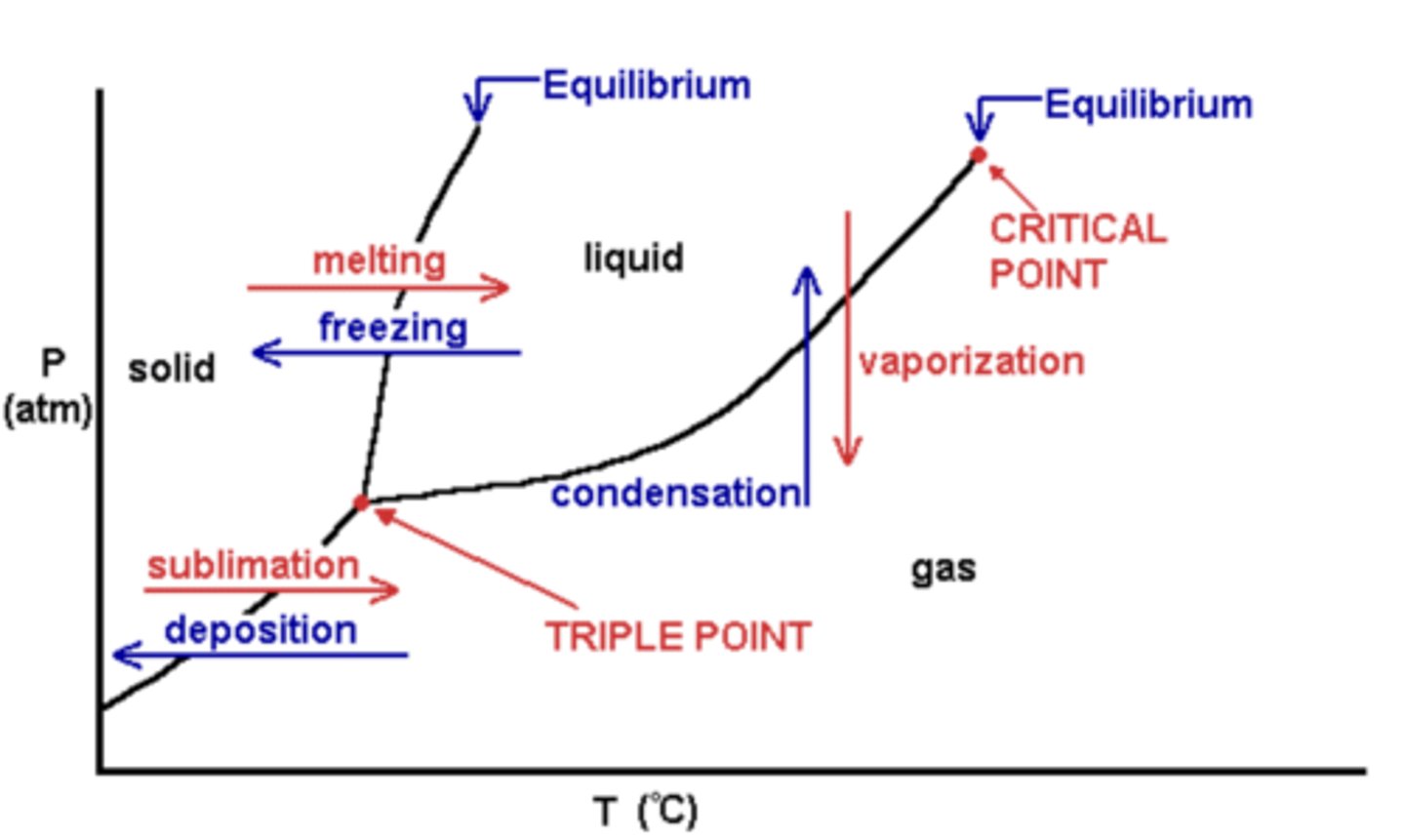
Pressure-Temperature Phase Diagram
Shows areas where the temperature and pressure combination will incur a solid, liquid, or gas phase.
The boundary lines are where two phases are present.
The triple point is a unique pressure and temperature where three phases coexist.
The critical point is where the distinction between liquid and gas cannot be made.
Note that it is possible to go around the critical point to seamlessly transition from gas to liquid (or vise-versa) without having both phases present at the same time
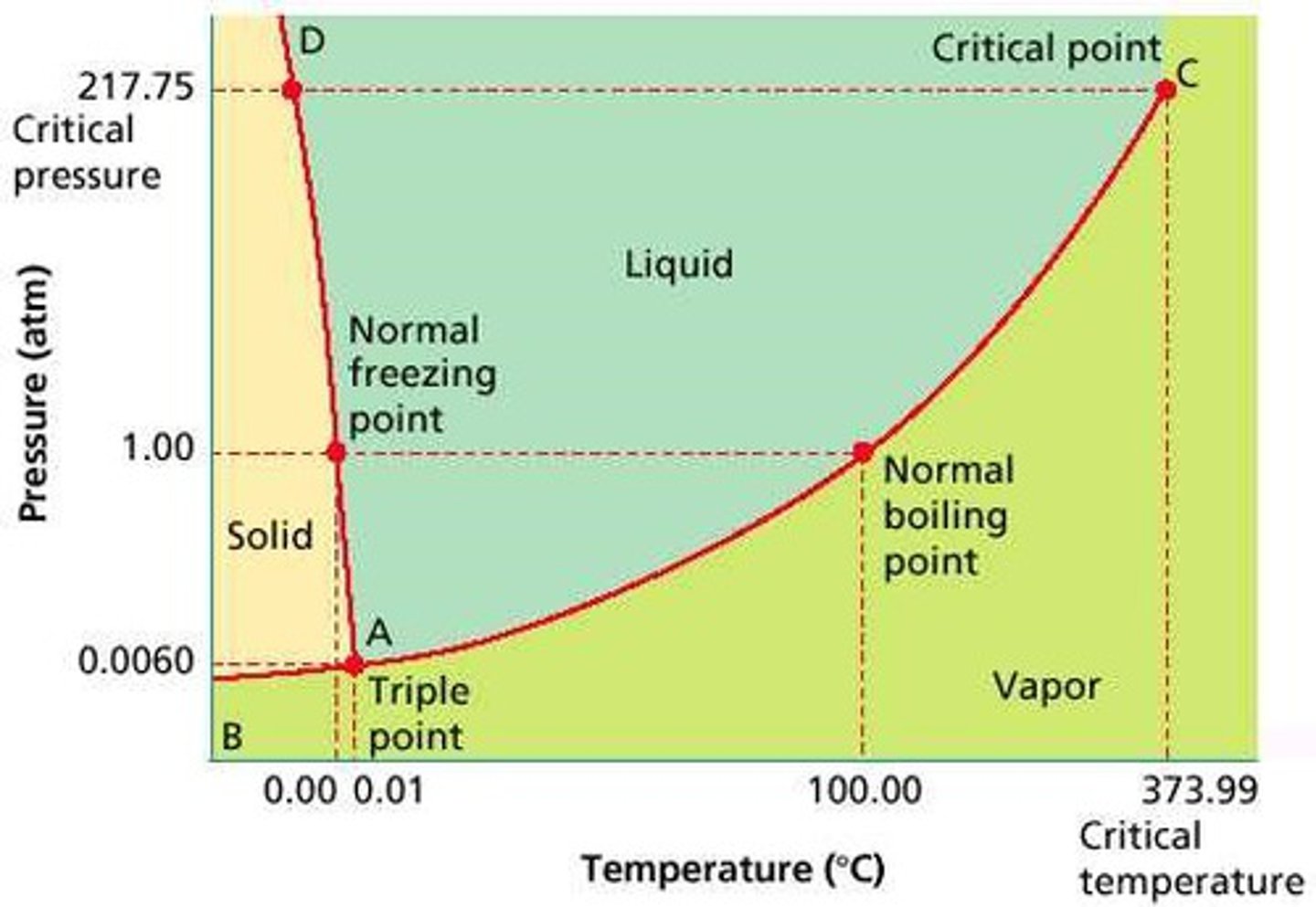
Pressure-Temperature Phase Diagram for Water and other Anamolous Molecules
Notice that the equilibrium line between the solid and gas phase curves slightly to the left, which means that the melting point will DECREASE as the pressure increases
Solution
A homogeneous (like, evenly distributes) mixture of two or more substances
Concentration
Basically how much of a solvent is in a solution
Molarity
It is the moles of solute over the volume of solution. Units are mol/liter

Molality
It is the moles of solute over the mass of the solvent (a bit more accurate than using volume because unlike mass, volume changes with temperature)

Binary Solution
Has one solute and one solvent
To go from a pure solute to a dissolved solute...
Solute-Solute bonds have to break
Solute-Solvent bonds are formed
Since there is more disorder in a solution than the independent solute/solvent, nature...likes it
Nonelectrolyte
Substance that does not ionize in water and cannot conduct electricity (ex: acetone)
Undergoes intact solvation; solute species don't have any bonds broken.
Strong Electrolyte
A substance that completely dissociates into ions when dissolved into water (ex: NaCl breaks up to Na+ and Cl-)
Weak Electrolyte
A substance that partially dissociates into ions when dissolved into water (ex: Acetic Acid --> gives up a H+ to the water)
Solution Stoichiometry
Basically a load of balancing equations, finding moles, volume, etc...
Pro tip: always convert to number of mols to make life easier.
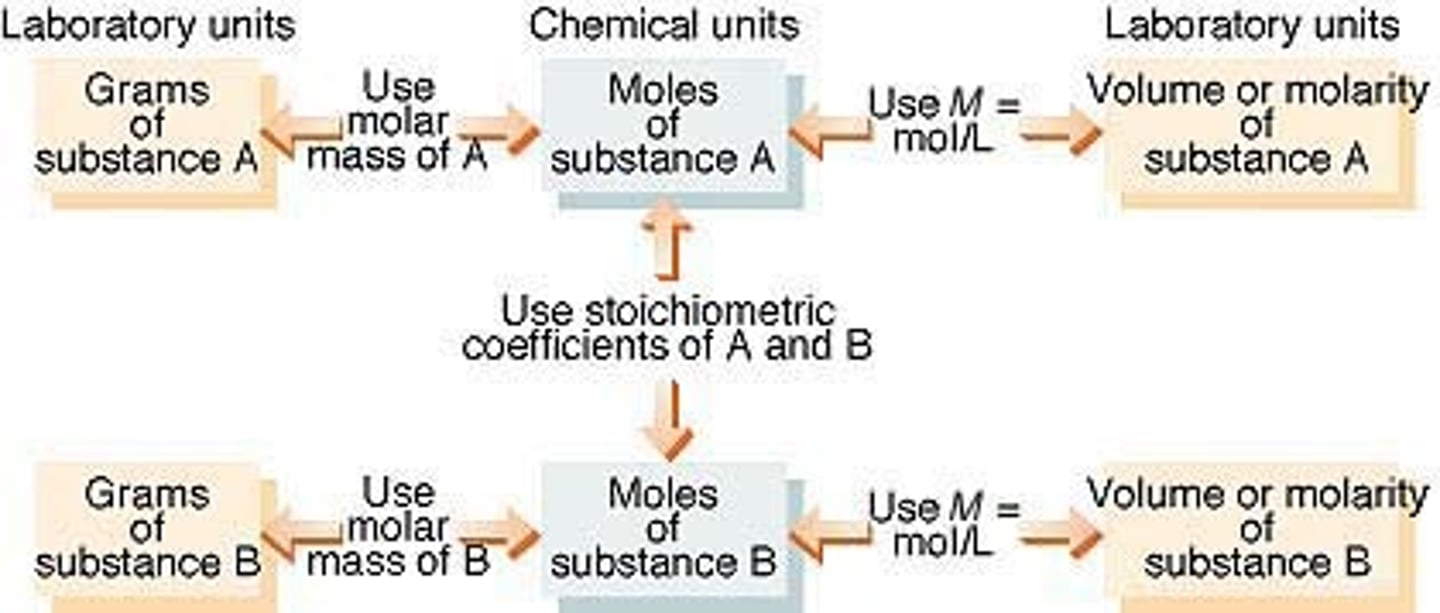
Titration
A solution of known concentration is used to determine the concentration of another solution.
End Point Indicator
A physical property that tells us that all of a reactant got consumed (ex: dye changes color)
Acid-Base Titration
A titration process where one solution is a base and another is an acid.
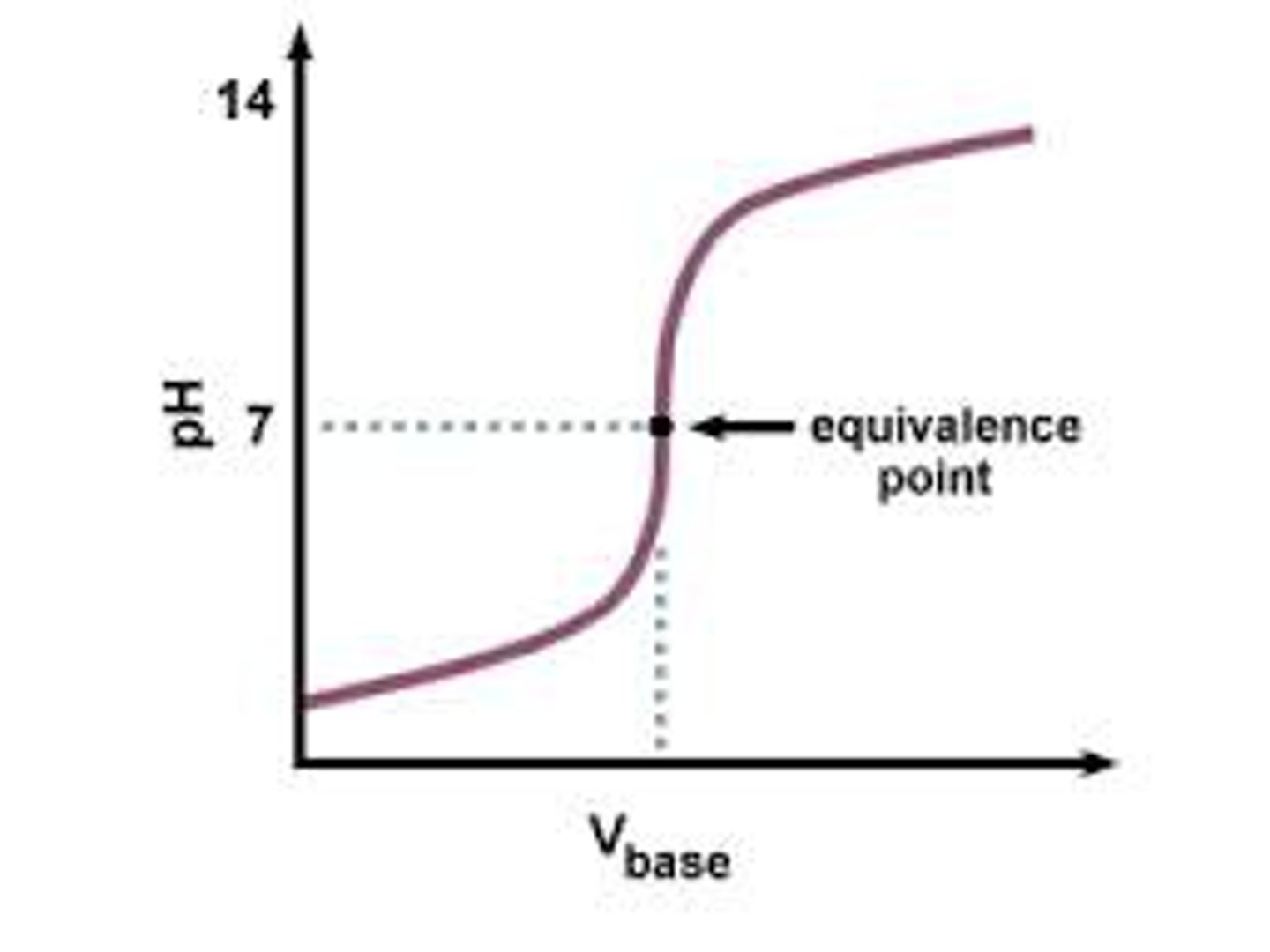
Requirements of acid-base titration
Strong acid and strong base
or
Strong acid and weak base
or
Weak acid and strong base
Strong Acid and Weak Base
A strong acid and a weak base will create an acidic solution. Some base will be left over because the acid will completely react. Will basically create a weak acid
The reaction goes to completion

Strong Acid and Strong Base
Will neutralize each other and create water
Weak Acid and Strong Base
A weak acid and a strong base will create a basic solution. Some acid will be left over because the base will completely react. Will basically create a weak base
The reaction goes to completion

Weak Acid and Weak Base
The reaction will not go to completion because neither the base nor the acid will completely react.
Please don't titrate with this
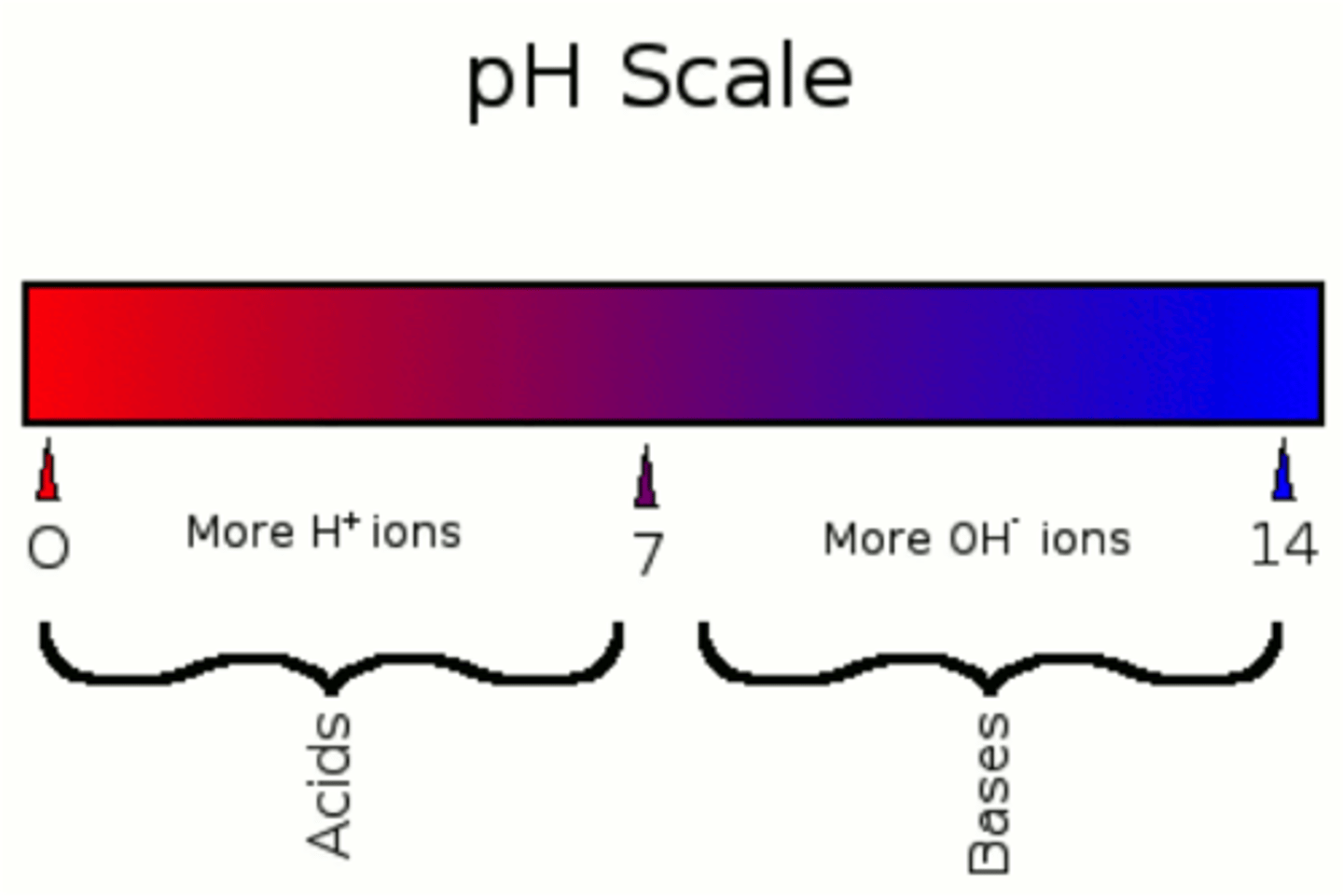
pH
The negative of the logarithm to base 10 of the concentration of hydrogen ions, measured in moles per liter; a measure of acidity or alkalinity of a substance, which takes numerical values from 0 (maximum acidity) through 7 (neutral) to 14 (maximum alkalinity).
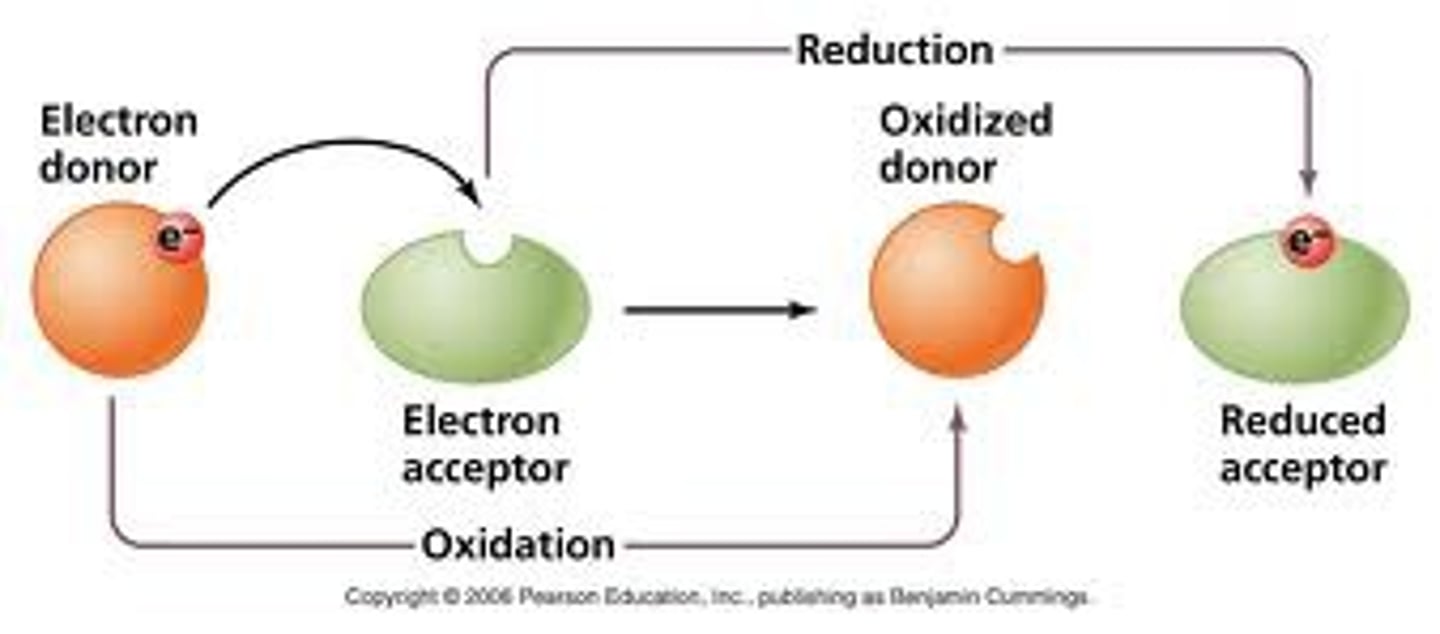
Oxidation-Reduction (Redox) Titrations
electrons are transferred between reacting species as they combine to form products.
Reduced species gains electrons
Oxidized Species gives up electrons
Steps to Balance Redox Equations
1. Write two unbalanced half equations (one for the reduced and one for the oxidized)
2. Insert coefficients to balance all elements except for O and H
3. Balance oxygen by adding H2O to one side of each half-equation.
4. Balance hydrogens by adding H2O to one side and H3O+ (for acidic solutions) or OH- (for basic solutions to the other
5. Balance charges of equations by adding electrons
6. Multiply equations to balance electrons then add them together
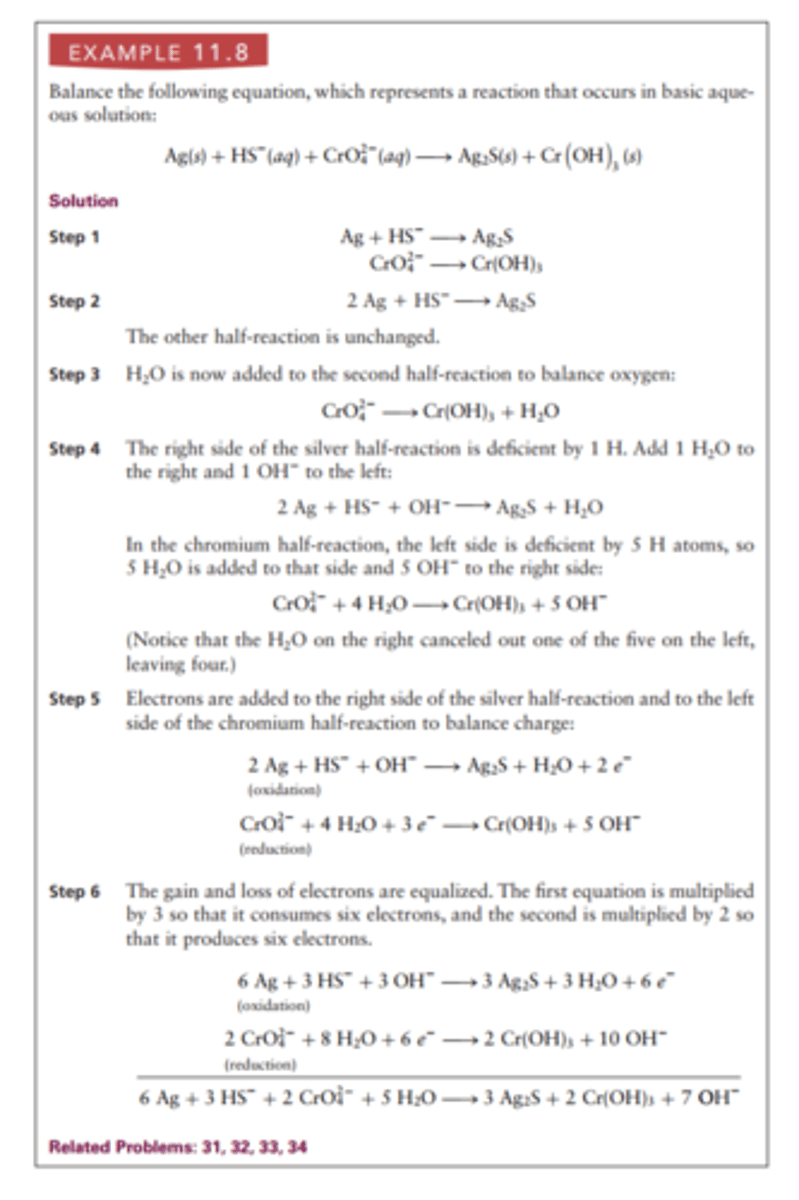
Oxidation Number
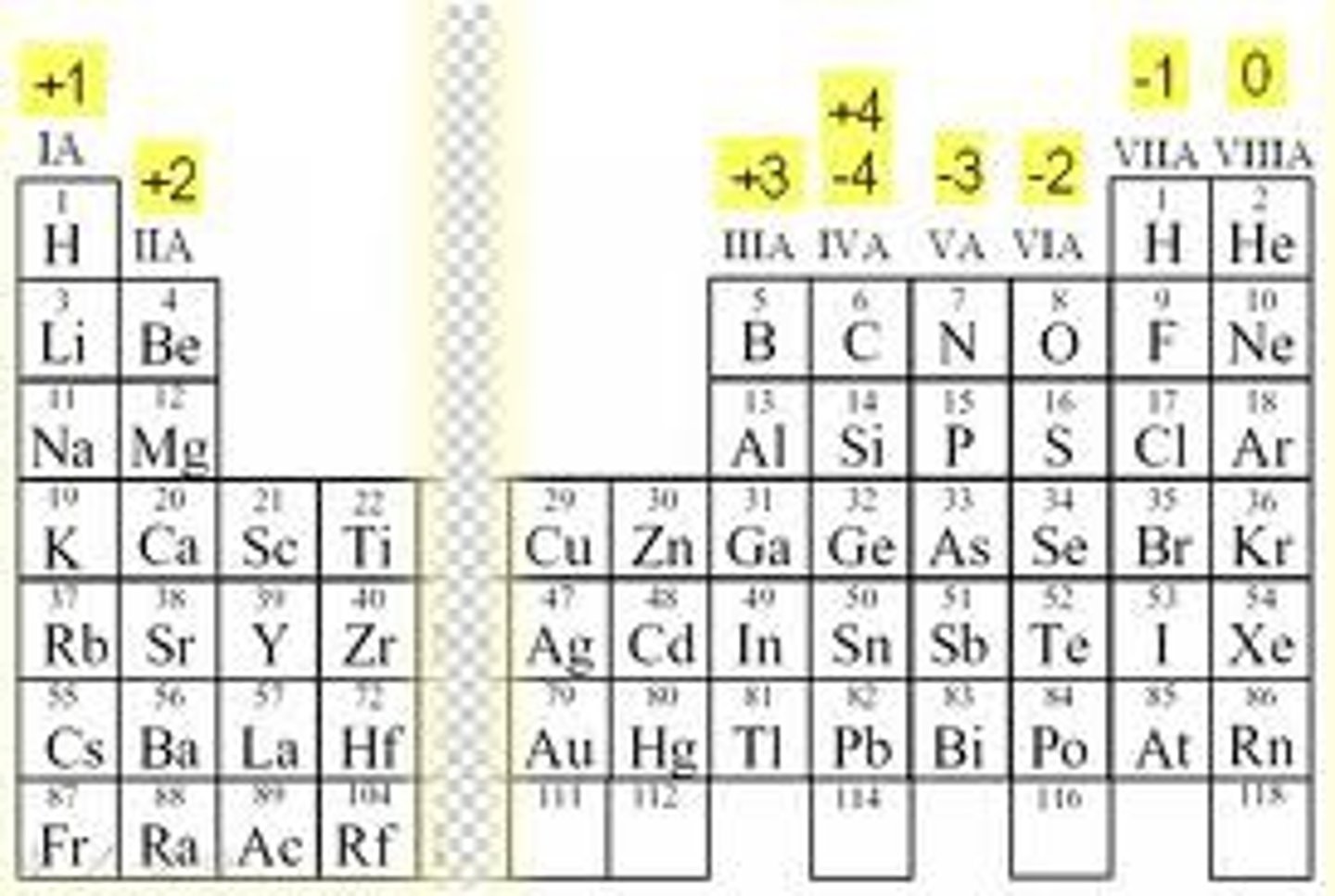
a number assigned to an element in chemical combination that represents the number of electrons lost (or gained, if the number is negative) by an atom of that element in the compound.
Colligative properties
The properties of a liquid that may be altered by the presence of a solute:
vapor pressure lowering
boiling point elevation
freezing point depression
osmotic pressure
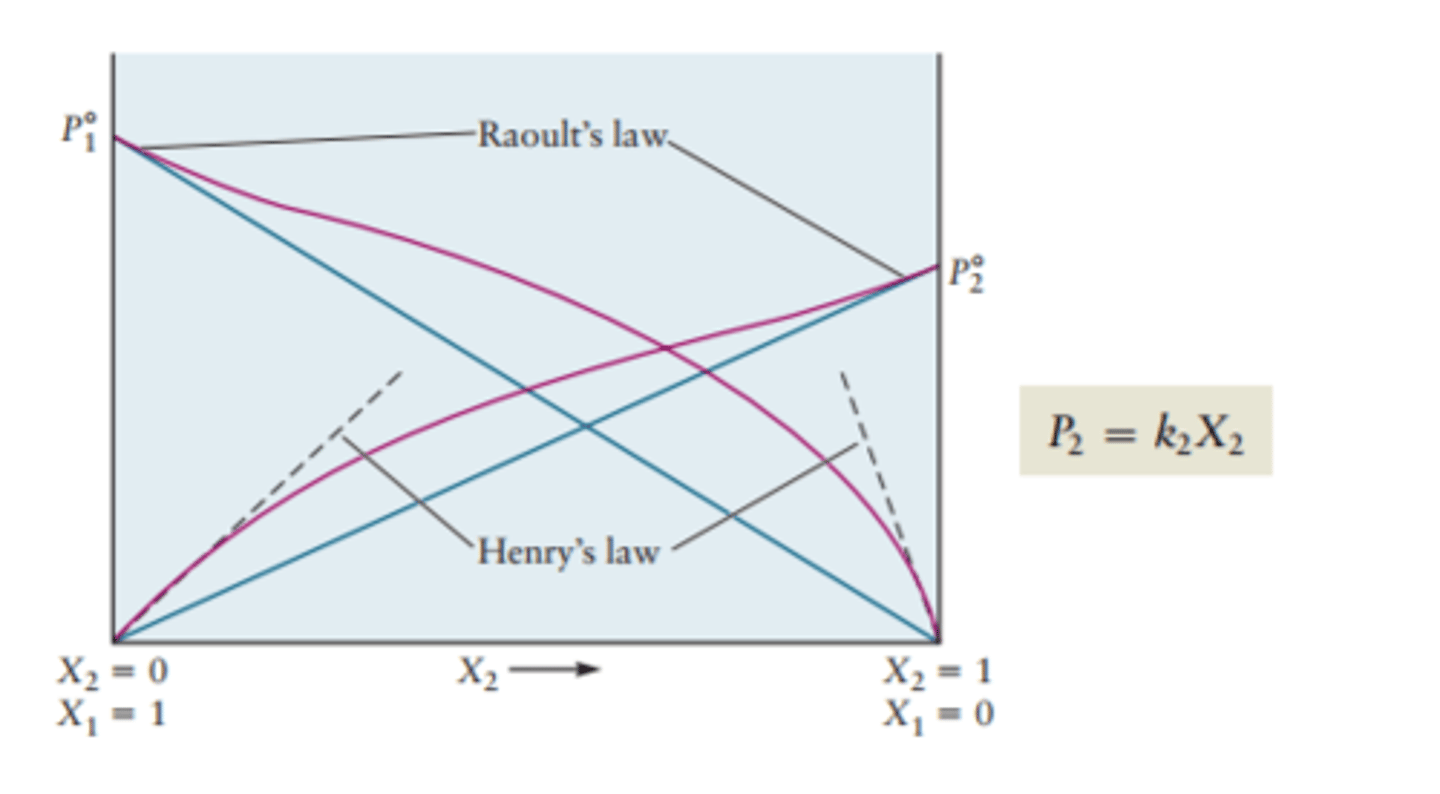
Raoult's Law
the vapor pressure of a solution is directly proportional to the mole fraction of solvent present
Psolution = Xsolvent x Psolvent
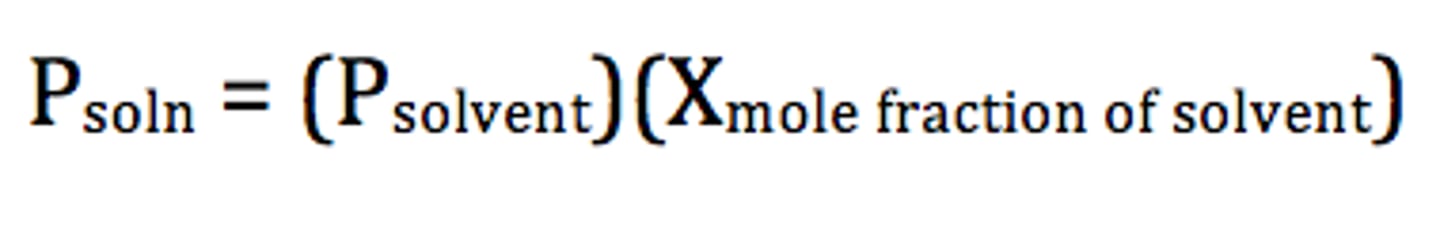
Vapor Pressure Lowering
The lowering of vapor pressure of a solvent by the addition of a nonvolatile solute to the solvent.
Note that adding more nonvolatile solution will always lower the vapor pressure
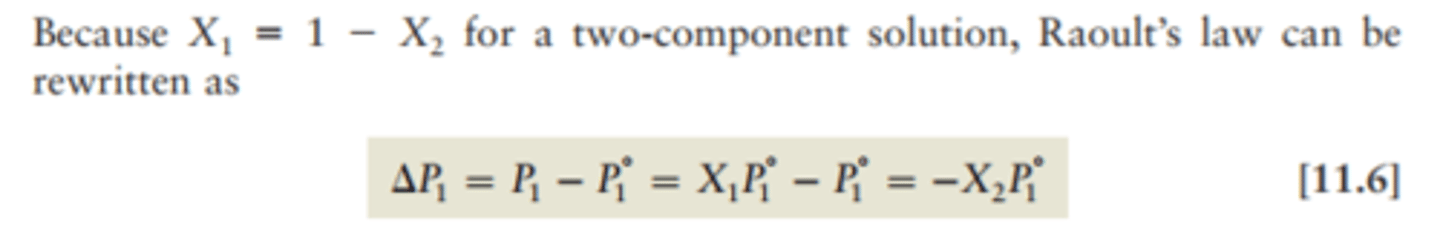
Boiling Point Elevation
The temperature difference between a solution's boiling point and a pure solvent's boiling point
Change in Tb = Kb x m
Note that Kb is a constant, and is not Boltzmann's constant. The little m is molality, measured in mol/kg
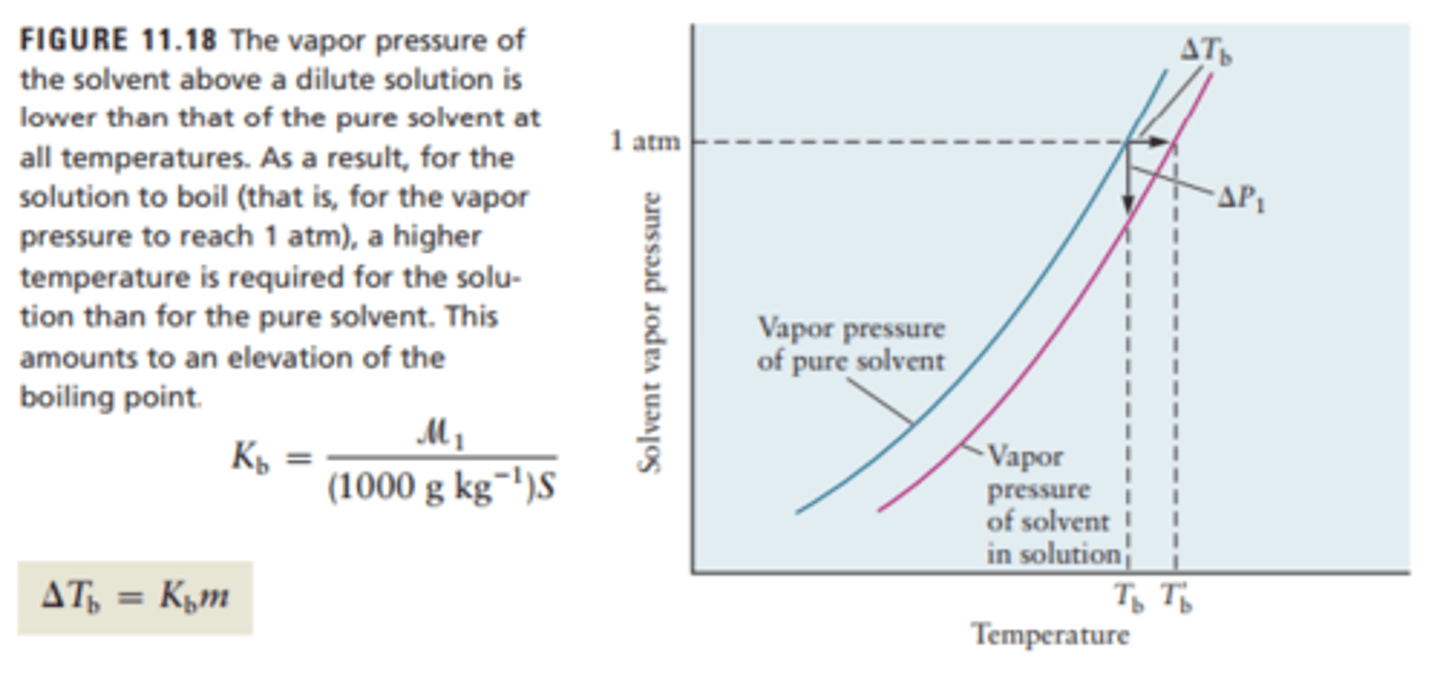
Freezing Point Depression
The difference in temperature from the freezing point of a solution (solute added to solvent) and the freezing point of the pure solvent
Change in Tf = -Kf x m
Kf is a constant that depends on the solvent and m is the total molality
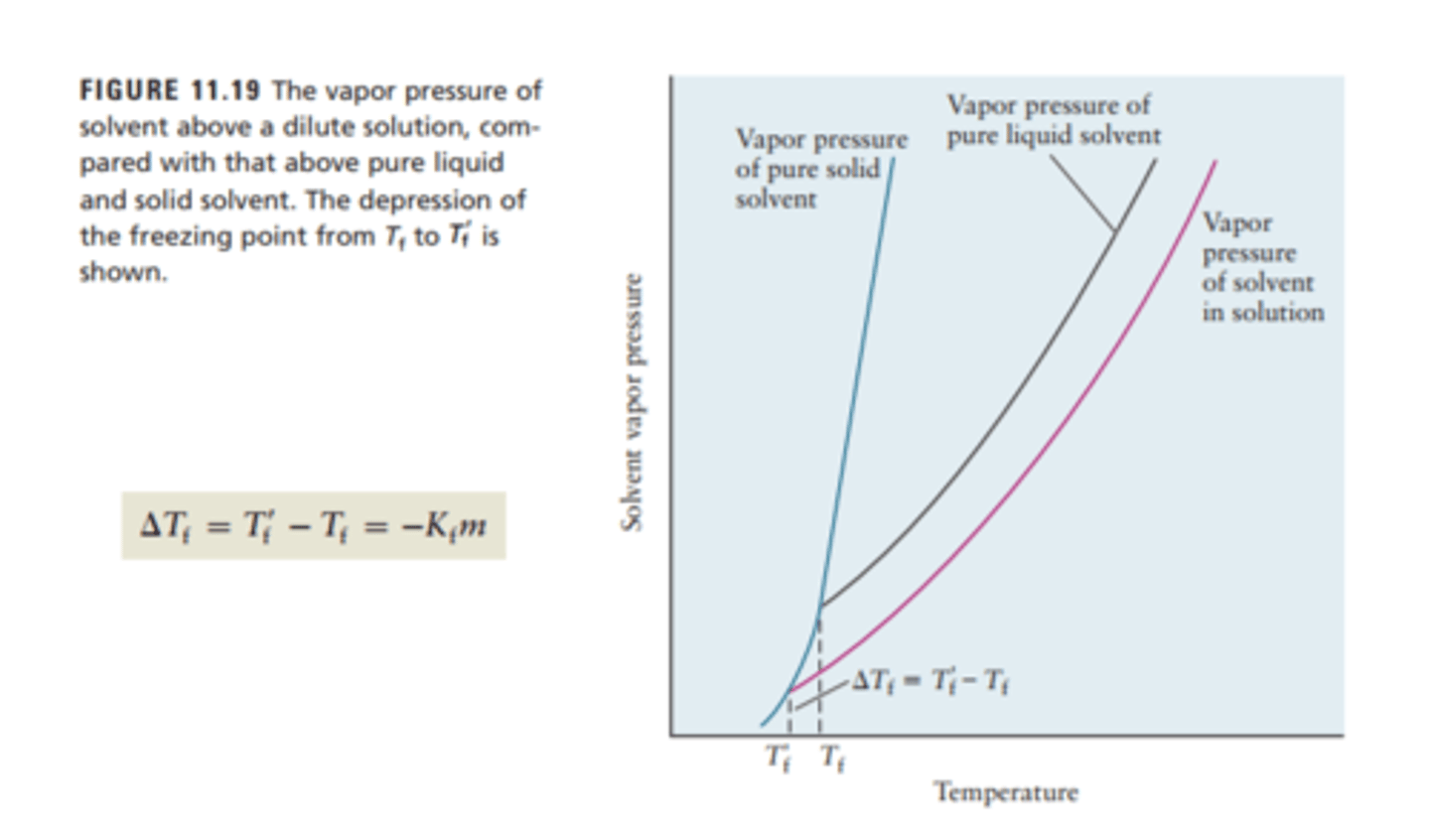
Osmotic Pressure
the external pressure that must be applied to stop osmosis

Osmosis
Diffusion of a solvent through a selectively permeable membrane
Henry's Law
direct relationship between pressure and solubility
S1/P1 = S2/P2
Bascially the pressure of molecule 2 is the ratio of the vapor pressures of molecule 2 and 1 (k) multiplied by the mole fraction of molecule 2 (moles of 2 over total moles)