pbhlth 162a final!!!!!!
1/702
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
703 Terms
what determines if infectious disease eradication is possible?
pathogen characteristics
no/limited non-human reservoirs
easily identifiable/clear diagnostics
emerging resistance to intervention
minimal susceptible population needed for pathogen to survive
mode of transmission
vector control
intervention effectiveness
efficient and practical interventions: vaccines
reduce and susceptible population significantly
technical and operational factors
practical diagnostics
effective surveillance systems
socionomic and poltical factors:
strong societal and political commitment
funding/cost effectiveness
if eradication is not possible, what should we aim for?
disease elimination in a specific area/population
disease control: reduce incidence, prevalence, morbidity, or mortality
targeted interventions: areas of highest disease burden and tailored approaches based on local conditions
addressing social and economic factors/strengthening health systems
where do emerging infectious diseases come from
viruses: mostly zoonotic infections
bacteria: mostly emergence of antibiotic resistance
emerging infectious diseases: viral pathogens
covid 19
crimean congo hemorrhagic fever
ebola virus and marburg disease
lassa fever
MERS-CoV
Zika
Rift valley fever
what should we do for emerging diseases?
building vaccine, clinical testing, and pre-clinical capacity
enhance global surveillance to detect human or animal outbreaks
establish communication structures
improved building design and operation to limit disease transmission
technology/workforce development/collaboration
vaccine development
antibiotic development
pathogen surveillance
epidemic modeling
education
pathogen surveillance
environmental and wastewater sequencing can provide real time information
easier and more accurate than relying on mass testing/self-reporting
endemic diseases
tuberculosis
respiratory infections
diarreal diseases
malaria
helminths (worms)
emerging/reemerging diseases
aids
mutidrug resistant TB
dengue
hanta (HPS)
lyme disease
ebola
leptospirosis
cholera in the americas
covid 19
key factors in endemic diseases
poverty
poor sanitation/hygiene
malnutrition
overcrowding
key factors in emerging diseases
international trade and travel
economic development and land use
deforestation
new roads, mines, plantations
contact with wild animals
ecological and climate change
behavioural and demographic cange
technology and industry
microbial adaptation (antibiotic resistance)
breakdown of public health
susceptibility: lack of immunity
the convergence model
factors influencing the outcome of microbial infections
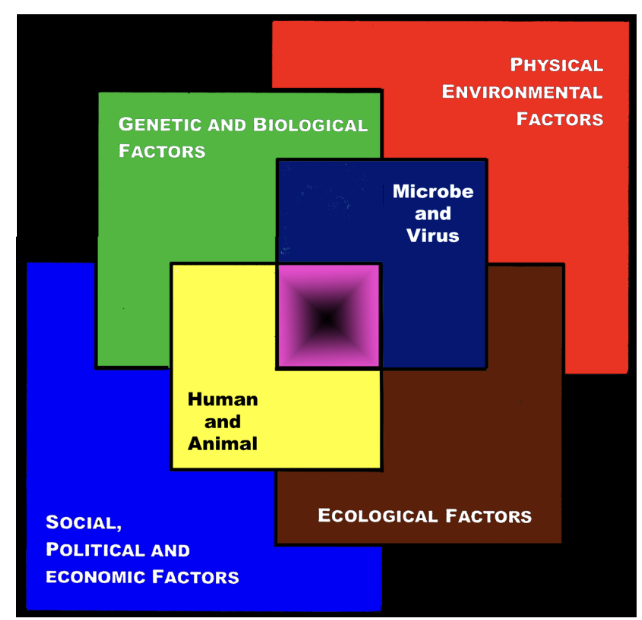
center of the box represents the convergence of factors leading to the emergence of infectious disease
black center represents unknown factors: “black box”
model indicates that all factors are interlocking
zoonotic infection
a human infectious disease originating from an animal reservoir and not requring the human as part of its life cycle
infectious agent/disease that can successfully circulate among animals without the host
diseases where human is required for life cycle are NOT zoonotic
helminths
other infections with animal intermediate
current zoonotic diseases may be due to…
more recent exposure of humans to microorganism
slower evolution of host-parasite relationship
Diseases that were zoonootic, but now exclusively human
small pox virus evolved from camelpox virus
measles virus from reinderpest virus of cattle
mycobacterium tuberculosis from M.bovis
HIV from SIV (simian immunodeficiency virus)
ebola virus
linear non-segmented ss - RNA genome
filovrius: appears as filamentous particles in the shape of a hook
first discovered near ebola river in Congo
ebola virus: natural reservoir
fruit bats in the Pteropodidae family are considered the natural host
ebola virus: transmission TO humans
transmitted to people from wild animals
close contact with blood, secretions, organs, or other bodily fluids of infected animals
in africa, infection has been documented through the handling of infected champanzees, gorillas, fruit bats, monkeys, forest antelope, and porcupines found ill/dead in rainforest
ebola virus: transmission AMONG humans
direct contact with blood or bodily fluids of an infected symptomatic person
exposure to objects (needles) that have been contaminated with infected secretions
viruses often spread through families and friends that come in close contact with infectious secretions when caring for ill persons
ebola virus: transmission in health care settings
hospital staff not wearing appropriate protective equipment: masks, gowns, gloves
lack of proper cleaning and disposal of instruments (needles, syringes)
inadequate sterilization of instruments reused
symptoms of ebola
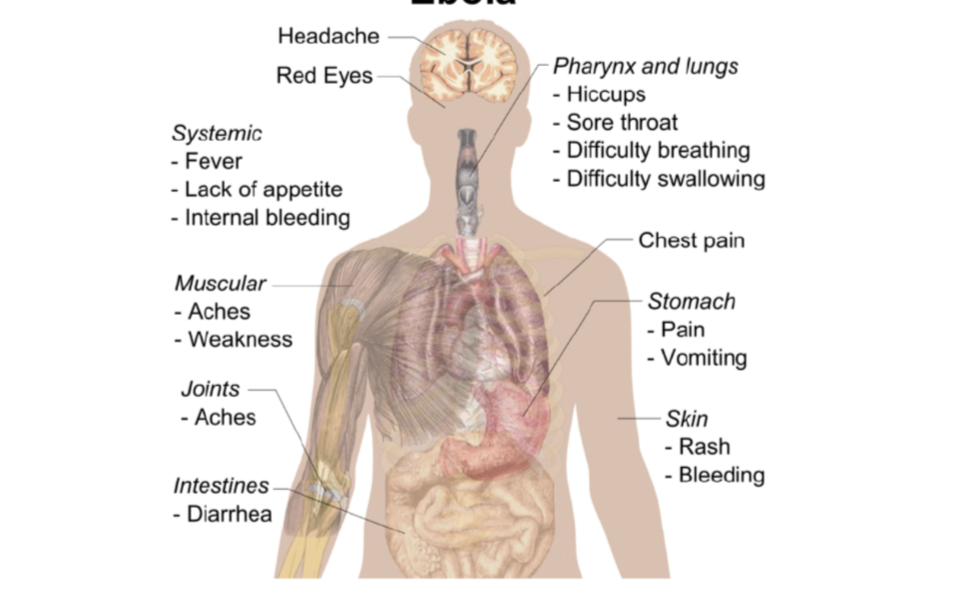
treatment of ebola
balance pateints fluids and electrolytes
maintaining oxygen status and blood pressure
treating for any complicating infections
monoclonal antibodies
ebola prevention
express ebola glycoprotein on surface of vesicular stomatitis virus (VSV), a benign virus that causes asymptomatic or mild flu-like symptoms in humans
rabies
ss - RNA, enveloped virus
rhabdovirus: rod/bullet shaped
most deadly infectious disease known
does not follow iceberg pattern
reservoirs of rabies
wild and domestic animals
rabid cat
fox
bat
mongoose
racoon
skunk
transmission of rabies
virus shed into saliva and transmitted when animal bites the human
enters via animal bite/skin break, replicates locally, migrates to neurones
may also be transmitted via exposure to bat feces
pathogenesis of rabies
virus multiples initially in tissue around bite
travels up nearby peripheral nerves to brain (1-3 months), then destroys cells in the CNS
moves to salivary glands
clinical manifestations of rabies
spinal cord, brain: acute encephalitis
infection to symptoms: 20-60 days
pain at site of wound: bat bites may be painless
neck pain around 2 months later
loss of control of movement
throat muscle paralysis: difficulty swallowing
drooling
hydrophobia (fear of water)
behaviour change: extreme agitation
coma
death 3 months after exposure
diagnosis of rabies
usually after death, using autopsy material from animal or human
negri bodies in the brain
PCR of brain / other material
samples taken from wound
fluorescent antibody test
PCR
treatment of rabies
no established antiviral treatment available
immunological treatment:
passive immunization injected
active immunization with inactivated vaccine; 2 injections in the arm, each 1 week apart
secondary prevention
virtually 100% effective
rabies prevention
animal control
required vaccinations of dogs
quarantine of imported animals in countries/areas that are rabies free
wildlife surveillance and reducing stray, unvaccinated dog and cat population
vaccination of people at risk of exposure
occupational
outdoor work with wildlife exposure
laboratory personnel
travelers to high risk areas
education of the public
toxoplasmosis
Toxoplasma gondii
protozoan parasite
Apicomplexa phylum
reservoir: cats and other feline
definitive host: cat
reservoir of toxoplasmosis
cats and other felines
transmission of toxoplasmosis
cats are definitive host: sexual cycle occurs only in intestine of feline family
transmission occurs from ingestion of material contaminated with cat feces
bird, rodents, ungulates, humans are intermediate hosts
T.Gondii forms cysts in their tissues
transmission can occur from eating undercooked beef
toxoplasmosis epidemiology
ubiquitous
invades all cell types
worldwide zoonosis
seropositivity increases with age
opportunistic infection associated with AIDS
clinical manifestations of toxoplasmosis
infection in most adults asymptomatic due to control by the immune system
infection severe and possibly fatal in immunocompromised individuals due to encephalitis, neurologic diseases
small children: fever, rash, pneumonia, encephalitis
toxoplasmosis effect on fetus
T.gondii can cross placenta in 40% of fetuses of non-immune mothers
mismarriage
12% of babies die shortly after birth
<20% are normal after age 4
damage to central nervous system
hydrocephaly
blindness
mental impairment
motor disturbances
diagnosis of toxoplasmosis
direct smear of material from spinal fluid or blood
ELISA serum antibody test
PCR
treatment of toxoplasmosis
sulfonamides, pyrimethamine, sulfadiazine (inhibit folic acid synthesis)
protozoans need folic acid in greater quantity than host cells
prevention of toxoplasmosis
avoid eating raw or undercooked meat
prophylactic antimicrobials for a non-immune pregnant woman who has been exposed
don’t have a cat, or have an indoor-only cat
if there is an indoor-outdoor cat
use hygienic food preparation practices
keep cats off kitchen counters and eating table
wash hands between handling cat and preparing food
be cautious in handling kitty litter
pregnant women should not empty kitty litter
effect of toxoplasmosis on mice
mice infected with toxoplasmosis lose their instinctive fear of the smell of cats
parasites effect may be permanent
toxoplasma and behaviour manipulation in animals
animals: greater predation of intermediate host by definitive host
increased non specific movements, more active
reduced neophobic behaviour (less fear of new scents, sounds)
reduced aversion (fatal attraction) to cat urine
reduced learning behaviours
toxoplasma and behaviour manipulation in humans
humans (chronic infection)
schizophrenia
suicide attempts associated with seropositivity to T Gondii
epilepsy
Congenital toxoplasmosis may reduce brain function
loss of psychomotor performance
men become more jealous, emotionally unstable, suspicious, short tempered, low self esteem
women and men more anxious
plague
Yersinia pestis
gram - cocobaccilius
reservoirs: praire, rat, chipmunk, squirrel
transmission of plague
requires the flea as a vector
bacteria multiply in flea gut and block it, causing flea to regurgitate infected material when flea feeds on a new host
flea is in a starving state so bites frantically
bubonic plague
Y. pestis multiplies in lymph fluid and lodges in lymph node nearest the site of flea bite
bubo = enlarged lymph node black from hemorrhage and fever occur within 2-6 days
disease not communicable among humans at this stage
pneumonic plague
occurs in 5% of plague cases
bacteria enter blood and disseminate to lungs, causing pneumonia
within 1-3 days skin becomes bluish to black from hemorrhage and lack of oxygen (black death)
disease is communicable among humans in this stage through respiratory route
mortality virtually 100% within a few days if early treatment not given
diagnosis of plague
patient sample from a bubo or gram stain (gram - coccobacillus)
direct smear made with laboratory antibody bacteria
rapid dipstick test for field testing of humans measures Y.pestis in human blood reacted with laboratory antibody to Y.pestis
treatment of plague
lancing/drainage of buboes
steptomycin, doxycycline, other antimicrobials
treatment effective if given early enough but diseased may not be recognized early enough
prevention of plague
surveillance for dead rodents in endemic areas
if surveillance shows positive animals, rodent extermination in residential areas
posting of warning signs
inspection of ships for rats to prevent transport to a new area
rat guards on mooring ropes
education of tourists not to feed squirrels and chipmunks out of their hands
use insect repellant outdoors in endemic areas
prophylatic antimicrobial after probable exposure
vaccination for people in high risk occupation
anthrax
bacillus anthracis
large, gram + bacilli, facultative anaerobe, endospore forming
endospores only form under aerobic conditions
zoonotic disease
herbivores: sheep, goats, cattle, reindeer - acquired for contaminated soil
carnivores infected from consuming meat
reservoir: animals (contaminated soil)
transmission of anthrax
human: contact with endospores during occupational exposure on farms/industries= wool, hides, meat, bones
wool sorters disease: respiratory anthrax
clinical manifestations of anthrax
cutanous anthrax: skin lesions, center black and necrotic
intestinal anthrax: symptoms mimic food poisoning
lesions/ulcerations in digestive tract
can lead to septicemia and death
outbreak from unpasteurized goats milk cheese
respiratory: most deadly; most concern with bioterrorism
symptoms similar to flu
inhaled into lungs, spores germinate in alveoli
phagocytized by macrophages, replicate
anthrax diagnosis
blood culture
gram stain
culture of external lesions
serological tests
PCR assays
treatment of anthrax
ciprofloxacin (fluoroquinolone)
penicillin
doxycycline
erythromycin
prevention of anthrax
human vaccine: 6 doses over 18 months
reduce exposure to endospocres
dispose of infected animals properly
vaccinate animals
one health triad
encompassing the collaborative goals providing optimal health for people, animals (domestic and wild) and the environment by considering interactions between all 3 systems
definitive host
an organism that harbors the adult, sexually reproducing form of a parasite
intermediate host
an organism that harbors a sexually immature stage of a parasite
biological vector
an arthropod that actively transmits pathogens that complete part of their life cycle within the organism
mechanical vector
vector in which the pathogen does not complete any part of its life cycle during transit
vector-borne disease vectors
ticks, mosquitoes, biting flies, fleas, blood feeding bugs, mites, lice
require a blood meal for their eggs to mature: proteins and nutrients in blood essential for egg production
feed in different ways: - piercing-sucking, tearing-rasping - suggests blood feeding evolved multiple times
molecules used by blood-feeding parasite
Anticoagulants: These molecules prevent the host's blood from clotting, making it easier for the parasite to feed. Parasites need to stop coagulation to ensure the blood remains liquid for feeding.
Vasodilators: cause blood vessels to dilate (expand), making it easier for the parasite to access blood. By widening the vessels, they increase blood flow to the feeding area.
Anesthetics: Parasites release these to numb the host so they don't feel the bite as much. This helps the parasite feed undisturbed, as the host won't notice the bite right away.
Immunomodulators: help parasites evade the host's immune system, allowing them to feed without triggering an immune response. By modulating the host's immune system, parasites can avoid detection and continue feeding longer.
targets for vector-borne disease
Human Host Interventions:
Anti-parasite/pathogen therapies: target the parasite or pathogen after it has entered the human body to reduce or eliminate the infection.
Vaccines: boosting the human immune response to the parasite/pathogen before or after exposure.
Arthropod Vector
Genetically modified vectors incapable of reproduction or pathogen transmission
Attractants/repellants and behavioral modifiers: Chemicals or tools that either attract or repel the vectors, making it harder for them to transmit diseases to humans.
Novel insecticides:
Vector longevity curtailers: Methods to shorten the lifespan of the vector so that they don't live long enough to transmit pathogens effectively.
Parasite/Pathogen Interventions:
Vaccines blocking parasite acquisition or transmission by arthropods: Vaccines that target the parasites within the arthropod vector, Insect immune regulators (smart sprays): substances that regulate or enhance the insect's immune system, potentially stopping the parasite/pathogen from being transmitted to the next stage of the life cycle.
general strategies to interrupt transmission of vector borne disease
vectors
Kill vector or alter vector competence for microbe
Inhibit feeding on humansń
Pathogen
Block transmission
Inhibit uptake
Humans
Vaccinate
Diagnose & treat
Reservoir
Eliminate reservoir
Vaccinate or treat reservoir
types of viruses
dengue
zika
west nile
dengue virus
Family: Flaviviridae
Genus: Flavivirus
+ssRNA virus
uncontrolled spread of dengue
Increase in numbers of cases
Geographic dissemination
Co-circulation of multiple serotypes
dengue epidemiology
spread of multiple serotypes
global trade and travel
urbanization
clinical manifestations of dengue
dengue fever:
high fever, headache, retro-orbital pain, fatigue, nausea, vomiting, cutaneous rash
dengue hemorrhagic fever/dengue shock syndrome (DHF/DSS)
increased vascular permeability, hemoconcentration, hypovelmic shock, hemorrhagic manifestations, thrombocytopenia, abdominal pain, cytoskin storn
sequential infection of dengue
while the body builds immunity to one dengue serotype after a primary infection, a secondary infection with a different serotype can lead to a more severe illness due to ADE, increasing the likelihood of complications like Dengue Hemorrhagic Fever (DHF) or Dengue Shock Syndrome (DSS).
Antibody dependent enhancement (ADE)
occurs when antibodies from a previous dengue infection worsen a secondary infection by helping the virus infect more cells, leading to more severe disease
integral hypothesis of dengue
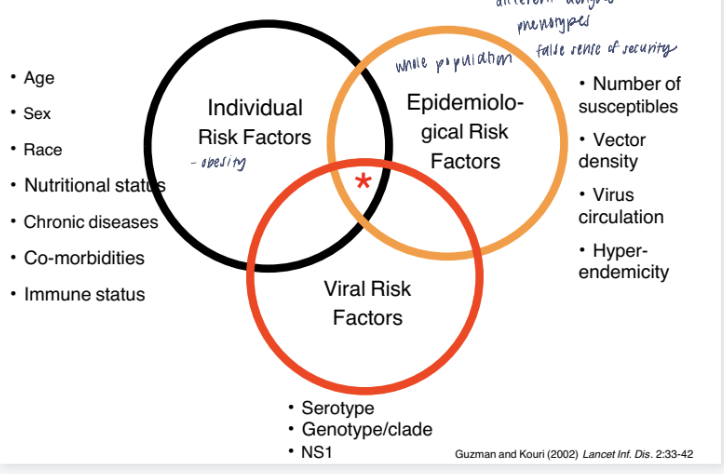
it’s not just one factor but the combination of personal characteristics, environmental conditions, and viral properties that determines the risk of dengue infection and its severity.
dengue diagnosis
Clinical
Serology
RT-PCR
dengue prevention
Mosquito control
Wolbachia – common obligate intracellular endosymbiotic bacteria of insects
disrupt/alter symbiotic relationship to affect vector competence (shorten mosquito lifespan, reduce level of pathogen, etc.)
Vaccine: challenging
Must include all 4 serotypes
Only licensed vaccine has safety issuesh
zika virus
ss(+) RNA virus
Belongs to family Flaviviridae; genus Flavivirus
spread by the bite of an infected Aedes species mosquito
can be passed from a pregnant woman to her fetus; Infection during pregnancy can cause certain birth defects
Local mosquito-borne Zika virus transmission in the continental United States
clinical presentations of Zika
onset of symptoms by Day 1 post infection, last until day 5
most pateints feel better after day 3
maculopapular rash, conjuncivitis, fever, arthralgia, and myalgia
rarely: vomiting, edema, myalgia
guillain-barre syndrome
microcephaly and congenital zika syndrome
viral encephalitis
inflammation of the brain
variety of viruses (togavirus, flavivrus)
west nile virus (WNV): widespread in USA
mosquito: biological vector
west nile virus
flavivirus
ss + sense
enveloped
spread throughout USA< multiple species of mosquitos, 60+ species of birds, mammals, reptiles, and humans
asymptomatic, west nile fever, encephalities
illness primarily in elderly and very young
life cycle: mosquito-bird-mosquito
viral encephalitis: prevention and control
surveillance
Blood screening
Mosquito traps/pool testing for viruses
Sentinel chicken flock immuno-serological testing
Dead bird testing for virus (esp. WNV)
ArboNET
mosquito control
eliminate standing water, mosquito fish, standing water
public education
larvicides
avoid mosquito bites
lyme disease epidemiology
30,000 cases reported to CDC every year; may be ~300,000 (clinicians, commercial labs)
Most cases in New England and Great Lakes
Seasonality: Late spring and summer in United States
lyme disease transmission
Borrelia burgdorferi
vector:
ixodes scapularis: new england and midwest
ixodes pacificus: west coast
need 48-72 hours to disseminate from tick to human
seasonal temporaility
most transmission in late spring and summer by nymph stage ticks
lyme disease: clinical
incubation: 3-32 days
stage 1: 70-80% pateints
erythema migrans (EM) rash
malaise
fatigue
fever
myaglia
stage 2: 5% of untreated pateints
weeks to months folliwng EM
neuritis, carditis, meningitis
stage 3: 60% of untreated pateints
weeks to years following EM
arthiritis, joint pain, swelling
lyme disease virulence factor
one of few pathogenic bacteria that can survive without iorn
enzymes use manganese, avoiding the problem many pathogenic bacteria face in acquiring iron
endoflagella: motility in viscous environment (mucosal tissue)
hide flagella antigens
lyme disease diagnosis
clinical
challenging in stage 2/3
isolation of B.burgdorferi (from captured tick)
significant change in IgM and IgG antibody
lyme disease treatment and prevention
antibitoics: only early after infection
EM: doxycycline/amoxicillin
neurological: IV antibiotics
prevention:
repellent and barriers
immediate tick removal
malaria etiology
phylum: apicomplexa
5 species of plasmodium
P falciparum: worldwide, most serious
P. vivax: rare in reuatorial africa, common in ammericas
P. malariae
P. Ovale. P. knowlesi
vector: anopheles sp mosquito
malaria epidemiology
majority of cases in africa (95%)
infants protected form infection by transfer of maternal Abs
young children at greatest risk of infection
non immune hosts at highest risk for complications and death: travelers, young children
attempts at malaria control
WHO’s worldwide eradication of malaria program (1957)
Widespread use of antimalarial drugs (e.g., chloroquine) in humans
Use of insecticide DDT to control the mosquito vector
Program failed to eradicate or even control malaria
Rise of parasites resistant to chloroquine, pyrimethamine, etc
Anopheles resistant to DDT
Now improved control methods; in 2018, Gates Foundationlaunched Malaria Eradication Program
Insecticide-treated bednets
ndoor residual spraying
Artemisinin combination therap
But, problems of resistance to insecticide and drugs
hypnozoites
in P. vivax and P. ovale a dormant stage [hypnozoites] can persist in the liver and cause relapses by invading the bloodstream weeks, or even years later
malaria clinical manifestation
High fever and chills (due to blood stage cycle)
Anemia (ruptured blood cells decrease oxygen transport)
Splenomegaly (spleen enlarges due to abundance of ruptured RBCs that needs clearing from circulation)
uniquely P. falciparum:
cerebral malaria: capillaries clog in the brain
renal failure: capillaries clog in kidney
pulmonary edema (fluid in lungs)
severe anemia
shock: excess antigen in bloodstream
why is p falciparum more virulent
can infect RBCs and erythropoetic (RBC) stem cels, exacerbating anemia
avoid clearance from spleen (survival strategy 1)
surface antigen in infected RBCs (pfEMP-1) binds adhesion molecules on endothelial cells in capillaries
PfEmp1 on RBC surface can bind platelets and other infected RBCs (rosetting)
by adhering to capillaries and rosetting, infected RBCs can clog blood flow to vital organs
where the capillaries are obstructed leads to particular clinical manifestations
p falciparum antigenic variation
survival strategy 2
P. falciparum evades the immune system by changing its surface antigens in a process called antigenic variation, leading to waves of immune responses.
parasite creates "waves" of antigen variation as each parasite clone switches its surface antigens, continuing this cycle until the parasite runs out of infected RBC surface antigens and matching endothelial cell receptors.
This strategy allows the parasite to persist in the host for a long time, even as the immune system gradually catches up to each variant.
Over time, after exposure to many variants, the host develops clinical immunity, allowing them to carry the parasite without experiencing symptoms, although the infection isn’t completely cleared.
treatment of malaria
chloroquine
resistance very common in P.falciparum infections
growing resistance in other plasmodium species
mefloquine
common chemoprophylaxis in travels
associated with weird dreams
antibiotics (doxycycline): used for chemoprophylaxis
arteminsins
used as herbal remedy in china for thousands of years
control & prevention of malaria
insecticide-treated bed nets
indoor residual spraying
chemoprophylaxis in travelers
repellents/long sleeved clothing
drain pools of standing water
vaccine: 3 doses + booster (1y)
low to moderate efficacy
relatively short-lived
onchocerciasis
etiology: enchocerca volvylus
filarial nematode
organism
adult worm
can live 15 years and release 700 microfiliare a day
vector
infected black of simulium sp
blackflies breed near fast-running water
onchocerciasis disease
microfilaria travel to subcutaneous tissue, mature into adults and reside in nodules
microfilaria in circulation travel to skin to be transmitted to biting fly
microfilaria migration causes extreme itching
lichenification
skin infections
sleep
microfilaria migration to eye causes blindess
immunopathogenesis of river blindness
4th leaving cause of blindness worldwide
microfilaria travel to corneal stroma and release wolbachia & wolbachia products when they die or release products
wolbachia LPS-like protein triggers macrophage and eosinophil chemotaxis to stroma and release cytokines
inflammation causes keratitis (corneal clouding) and blindess
wolbachia
Endosymbiotic bacteria in both adult worms and microfilaria
Required for embryogenesis
Can use drugs (e.g., doxycycline antibiotic) that target the Wolbachia and kill the adult worm)
onchocerciasis diagnosis
Skin snips for microfilaria
ID adult worms in nodules