Topic 1.1: Structure of Water and Hydrogen Bonding Diagram | Quizlet
1/19
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
20 Terms
Science Practice Skill: 2.A
Describe characteristics of a biological concept, process, or model represented visually.
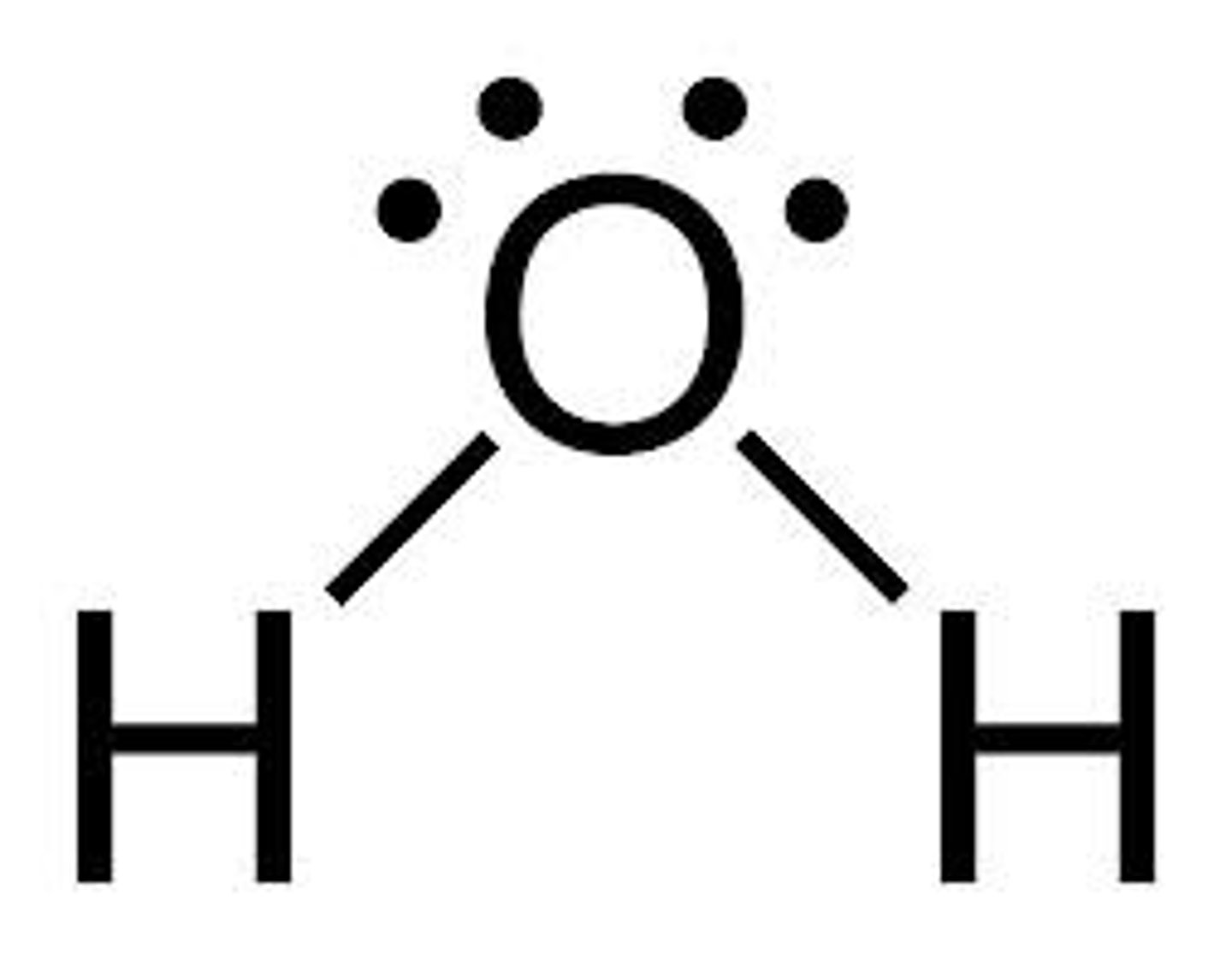
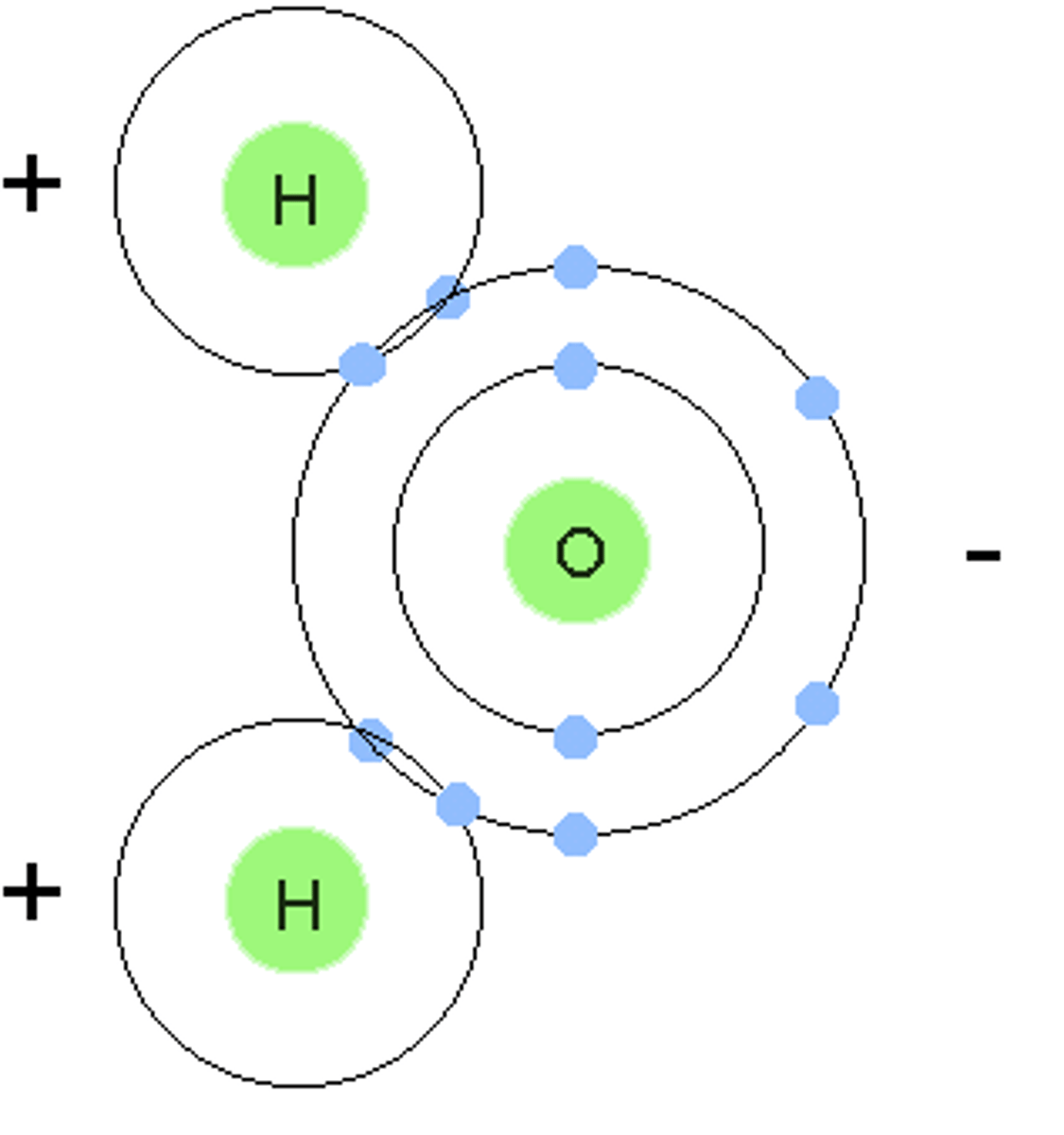
Water (H2O)
Composed of oxygen and hydrogen in a 1:2 ratio
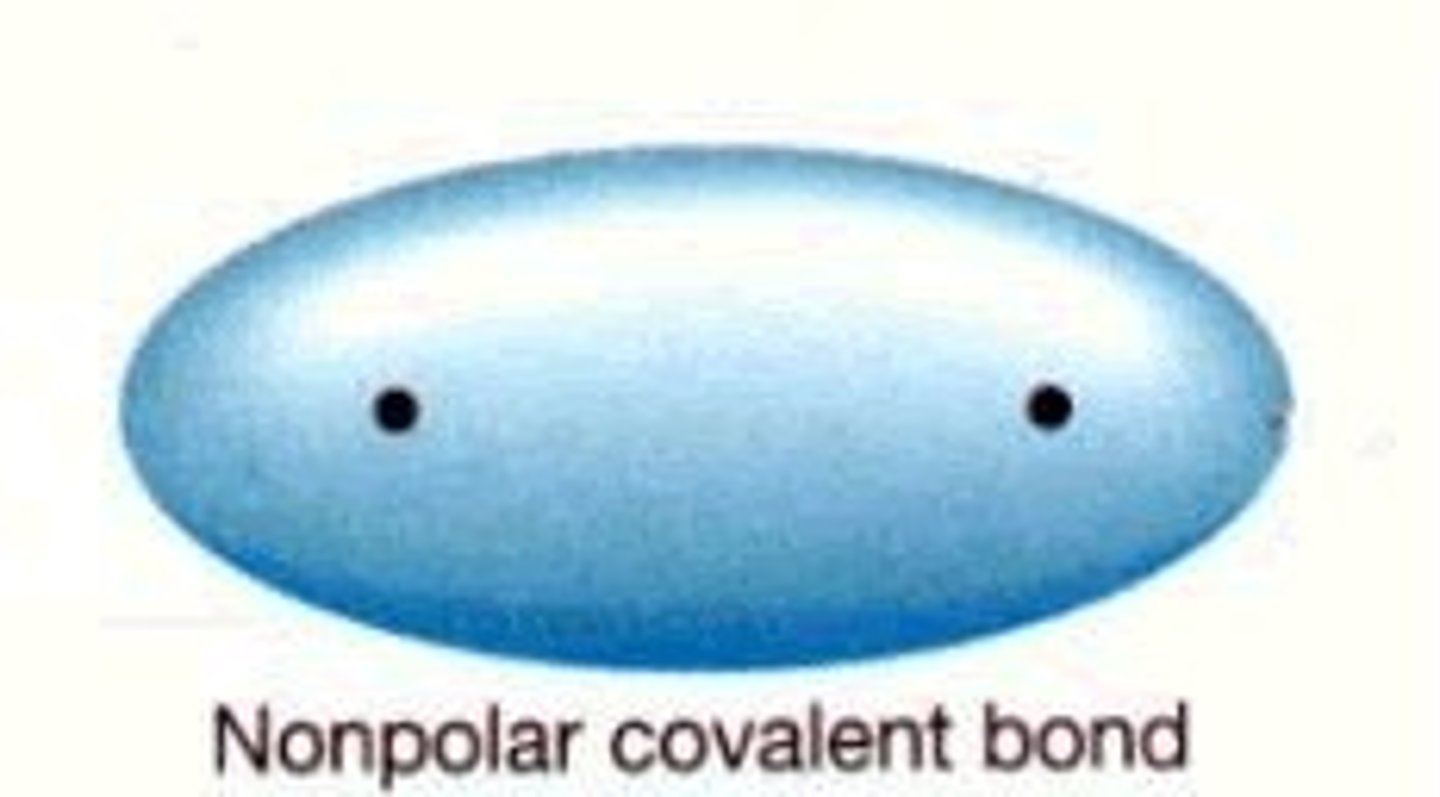
covalent bond
A chemical bond that involves sharing a pair of electrons between atoms in a molecule
Molecule
two or more atoms held together by covalent bonds
Compound
A substance made up of atoms of two or more different elements joined by chemical bonds
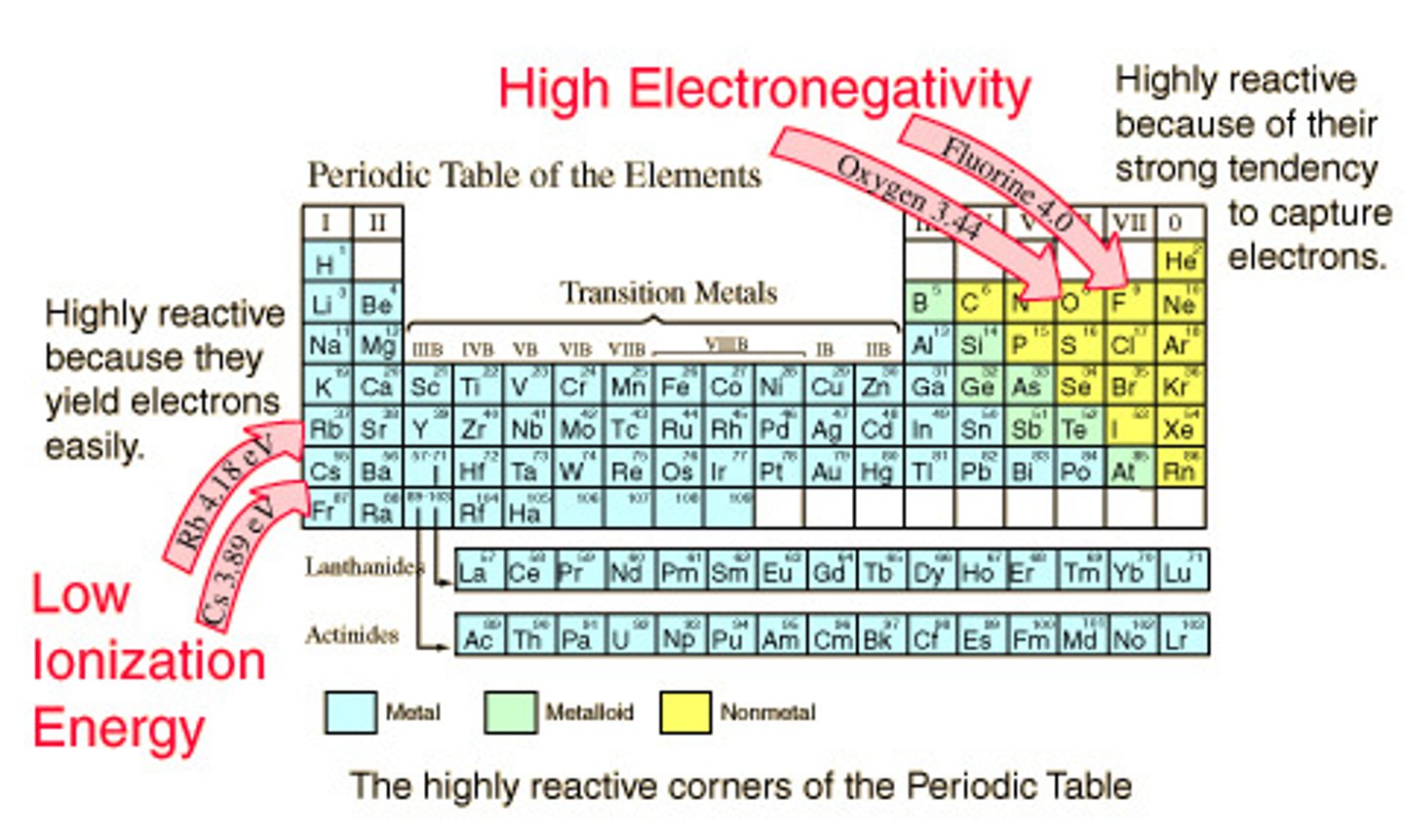
Electronegativity
A measure of the ability of an atom in a chemical compound to attract electrons
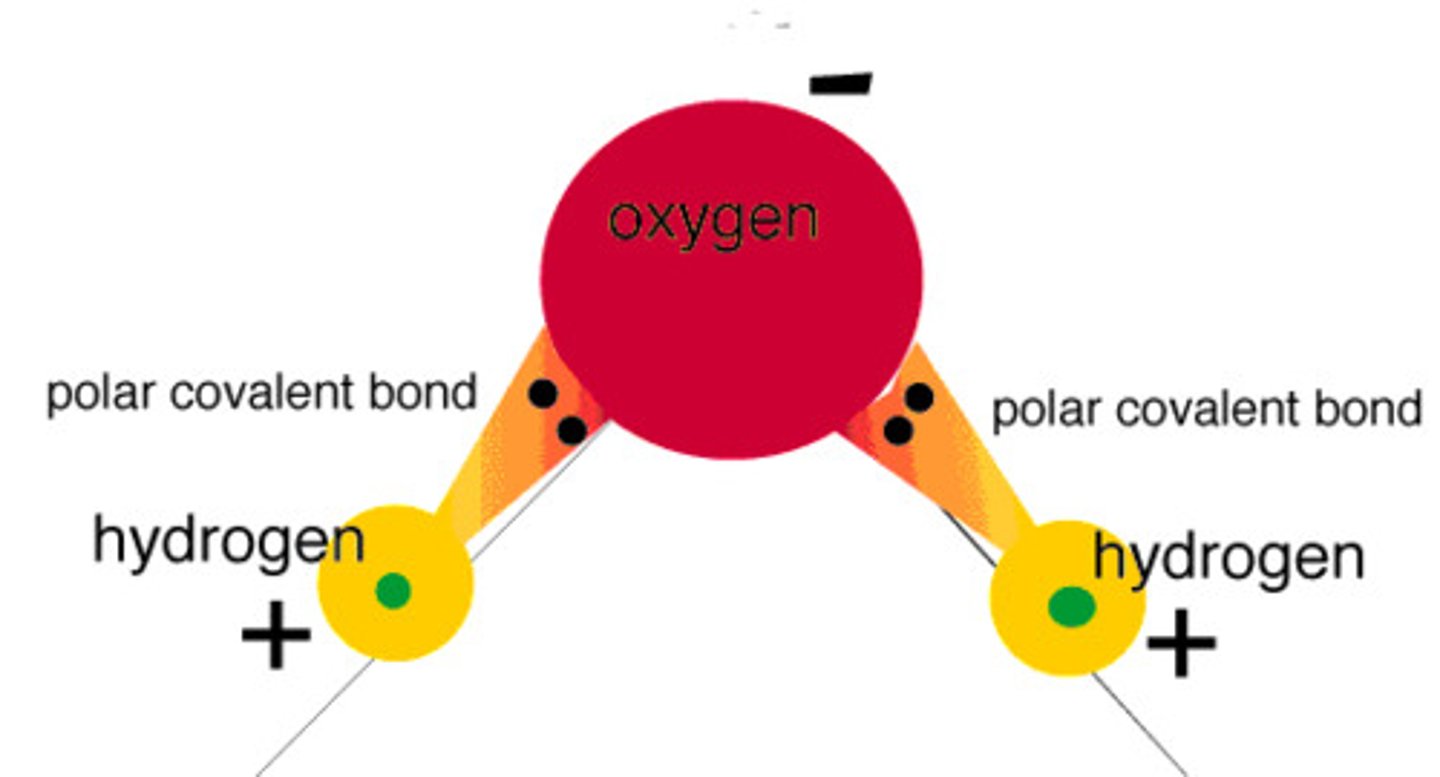
polar covalent bond
A covalent bond between atoms that differ in electronegativity. The shared electrons are pulled closer to the more electronegative atom, making it slightly negative and the other atom slightly positive.
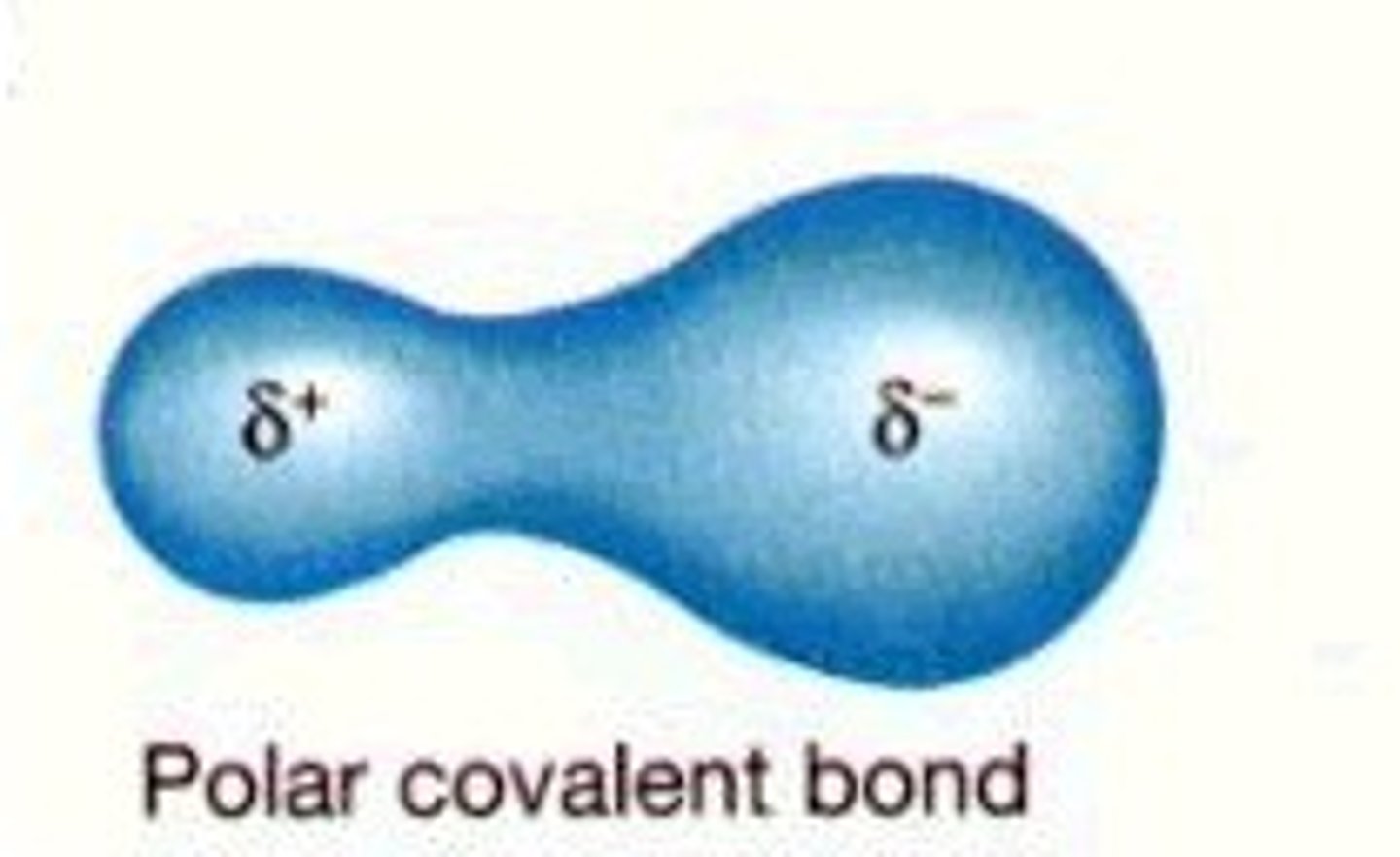
polarity of water
has polar covalent bonds; H is partially positive, O is partially negative
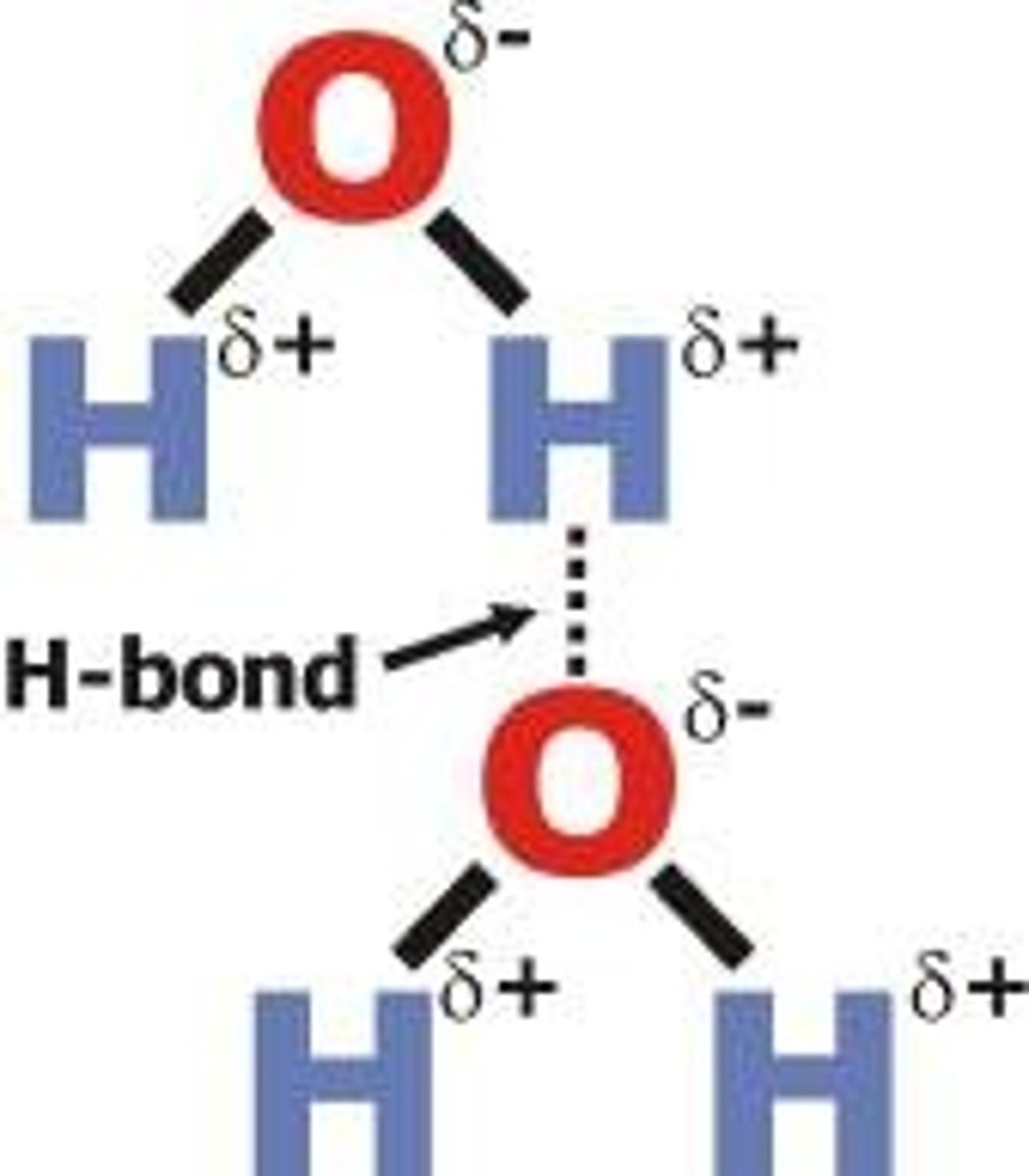
hydrogen bond
weak attraction between a hydrogen atom and another atom
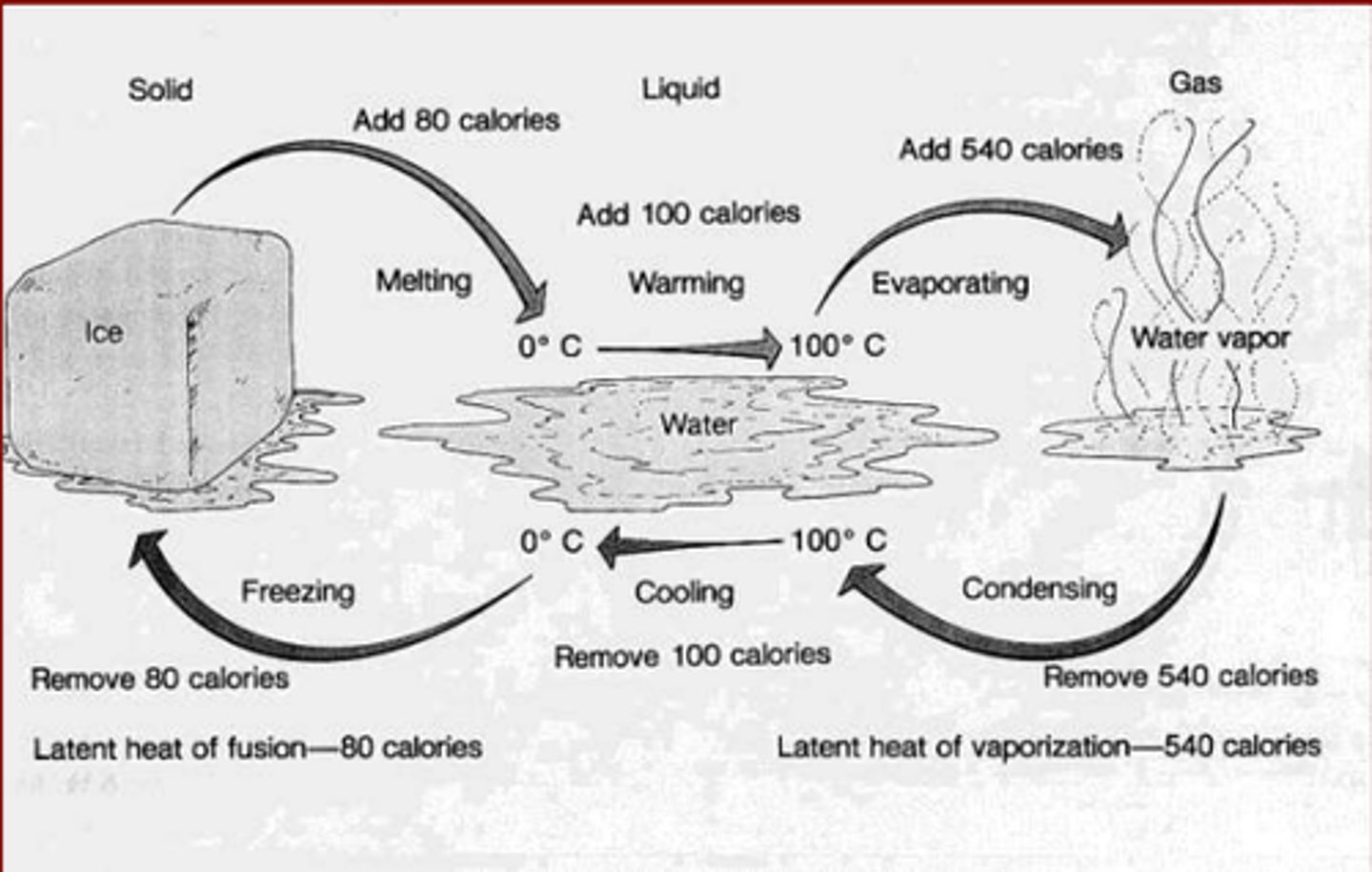
Properties of water
performs cohesion, performs adhesion, is an excellent solvent, has a high surface tension, floats in a solid state, has a high heat capacity, and performs capillary action.
cohesion of water molecules
Cohesion means water molecules stick to each other. Cohesion results from hydrogen bonds.
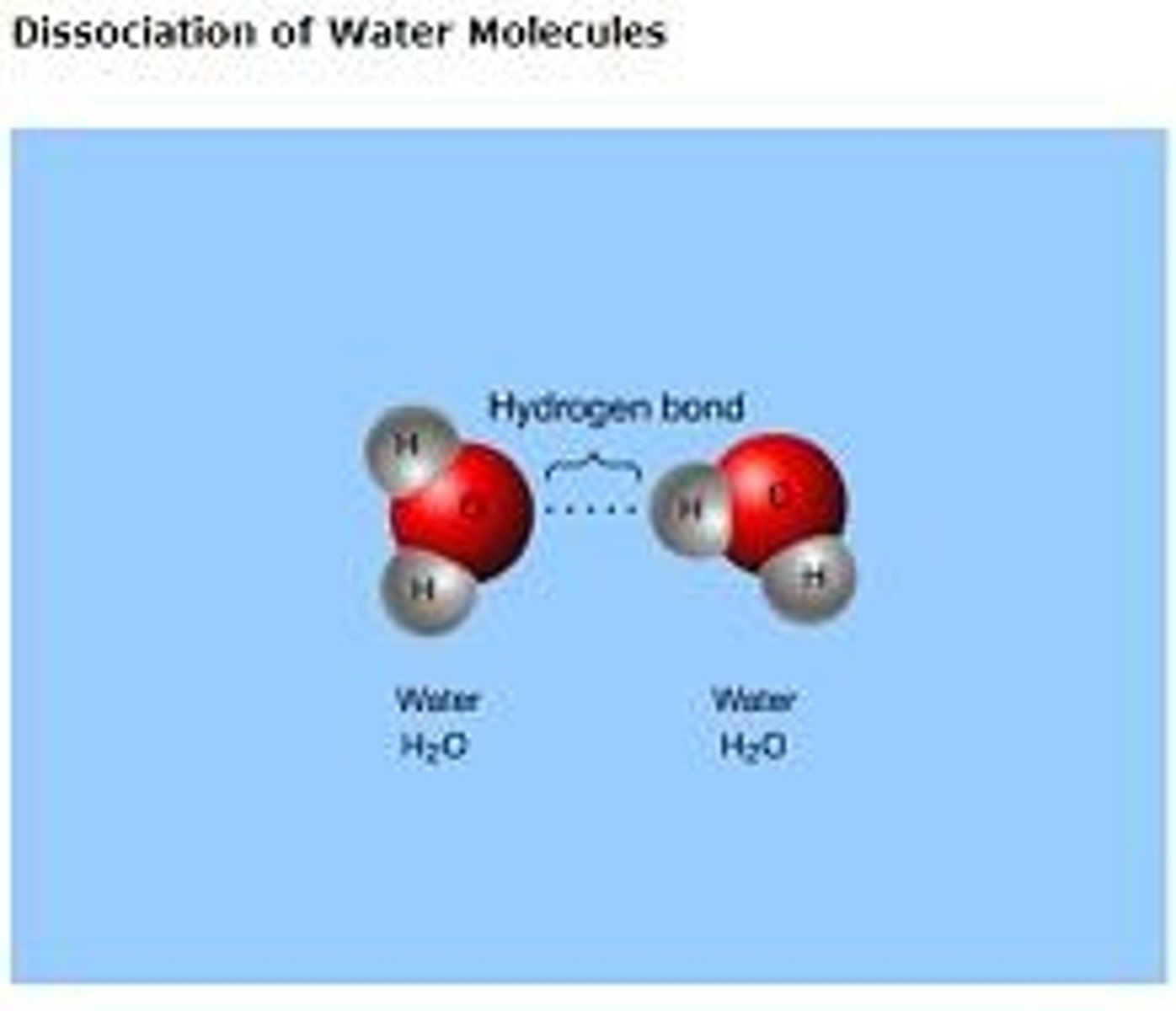
adhesion of water molecules
The clinging of water molecules to another substance as a result of hydrogen bonding

surface tension
A measure of how difficult it is to stretch or break the surface of a liquid
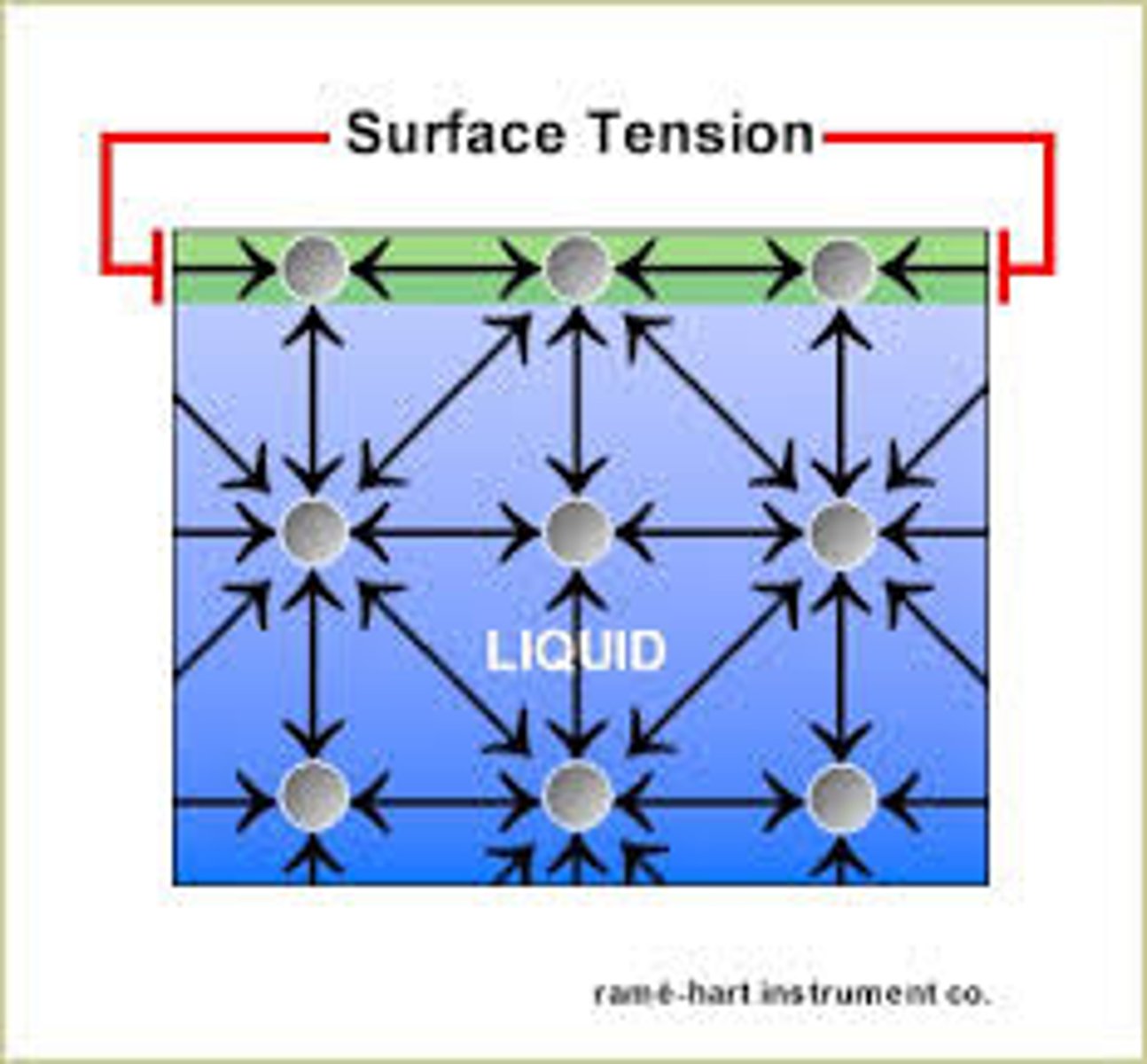
water has a high surface tension
results from intermolecular hydrogen bonds between molecules of water at the surface. allows insects to remain on the surface

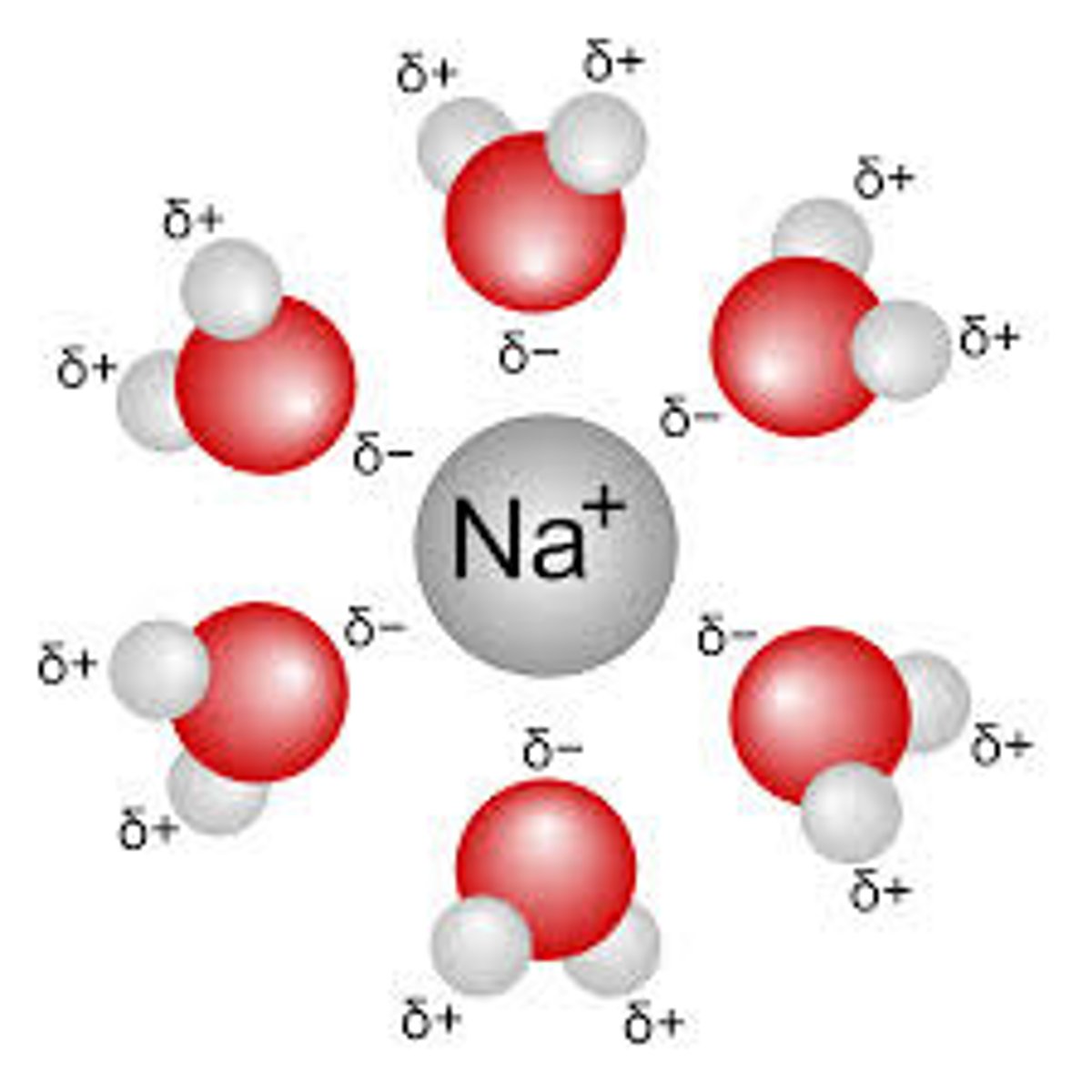
Water is an excellent solvent
Many things dissolve in water. Water's polar charges can keep other ions separated in solution
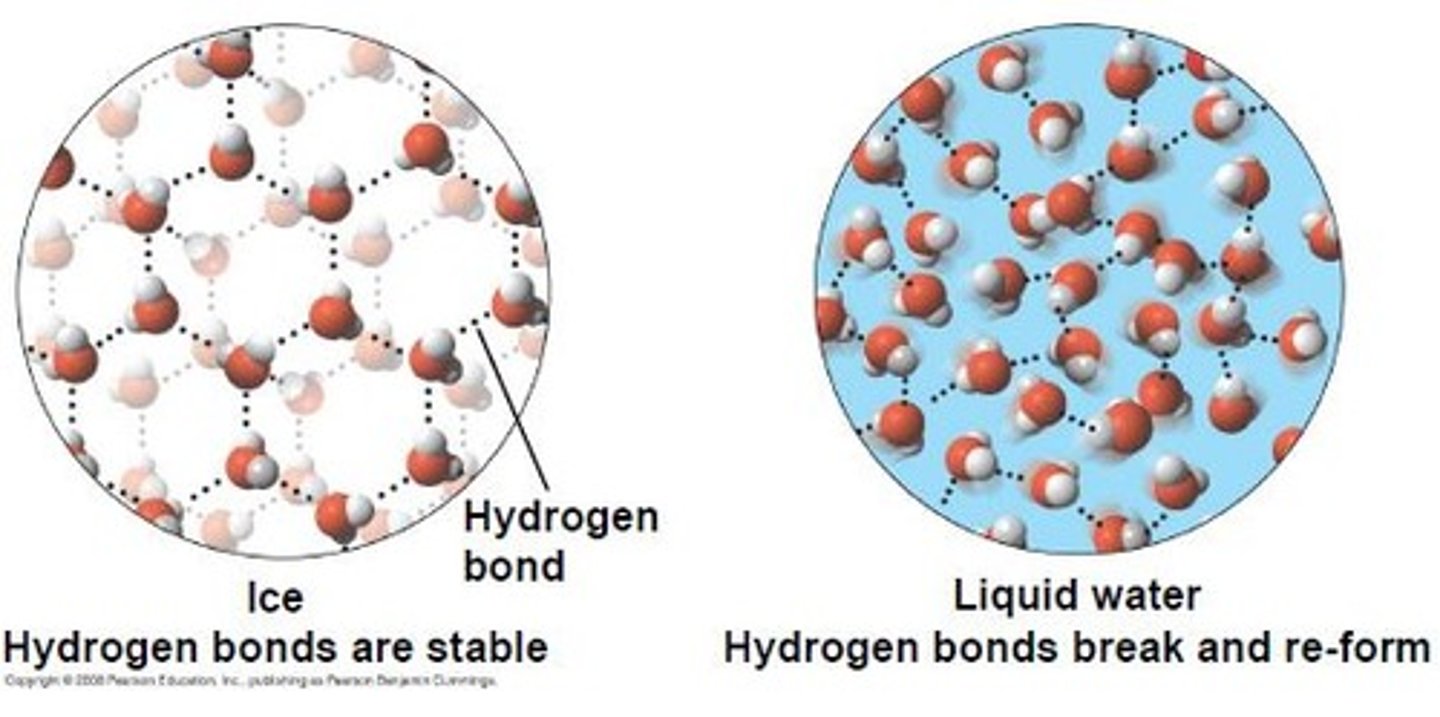
ice floats
Because molecules of water are farther apart in ice than in liquid water. This makes ice less dense that liquid water. This allows for organisms to live underwater in frozen climates.
Water has a high heat capacity
it can absorb large amounts of energy with only a slight change in temperature. This maintains organisms environments.

Water performs capillary action
Movement of a liquid through/along another surface despite forces such as gravity. Allows water to move out of roots of a plant and up into its stems and leaves
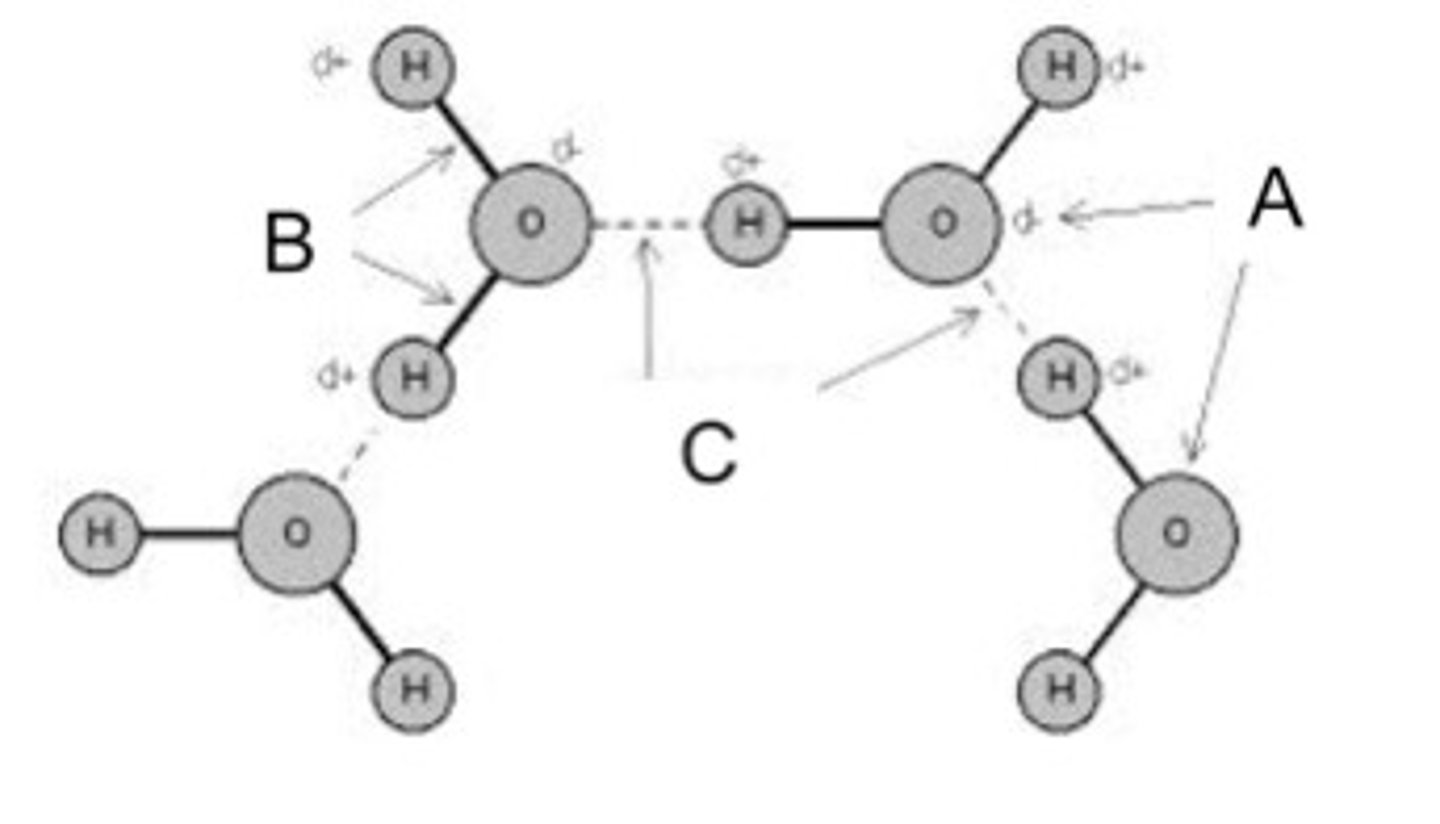
Identify the hydrogen bond(s) in the pictured water molecules.
C
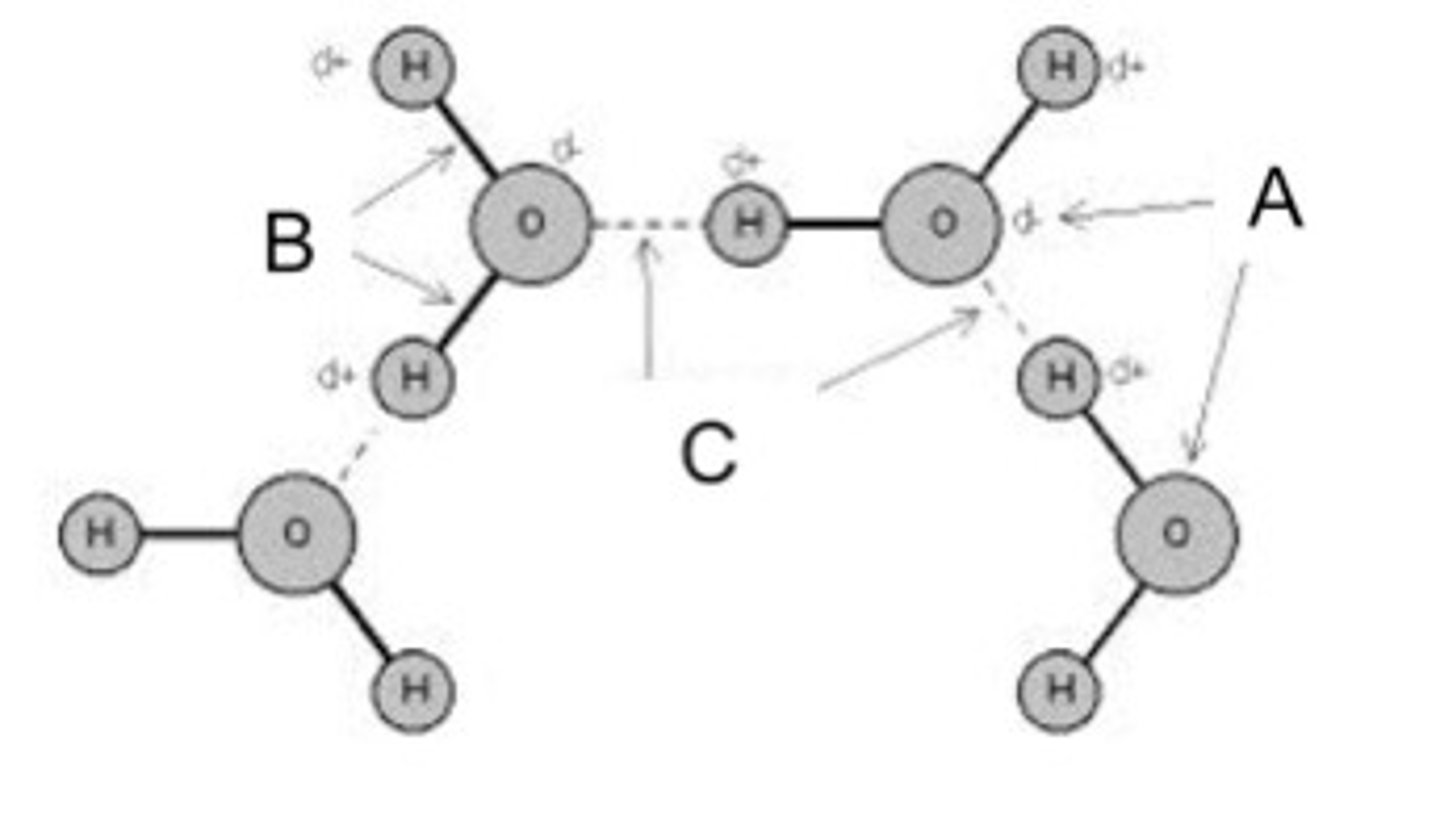
Identify the polar covalent bond(s) in the pictured water molecules.
B