1.1 Formulae Equations and Amounts of Substances
1/12
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
13 Terms
What is a molecular ion?
Two or more atoms covalently bonded with an overall charge
How do you turn a full equation into an ionic equation?
Split apart any ionic compounds that are in solution
Remove any spectator ions.
What are the state symbols?
Solids =(s) Liquid =(l) Gas =(g) Aqueous Solution =(aq)
What is Avogadro constant?
The number of atoms in 12.000 g of carbon-12
What is a mole (n)?
The amount of a substance that contains the Avogadro constant of atoms, molecules or groups of ions.
What is molar mass ( RFM /mr)?
The mass of one mole of a substance
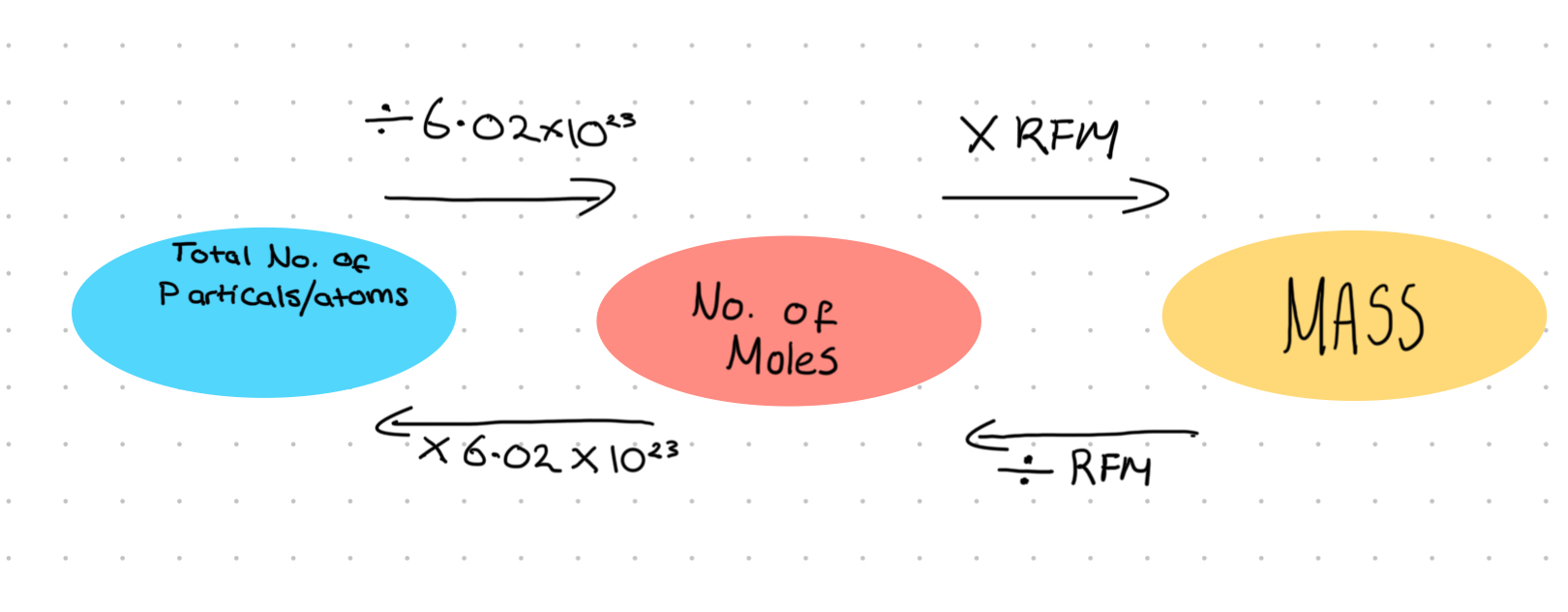
Diagram to convert number of particles /atoms in to moles and in to mass
What does anhydrous salt mean
A salt which contains no water of crystallisation
What does hydrated mean?
A salt which contains water of crystalisation
What is water of crystalisation
Water chemically bonded within a crystal structure
How do your calculate the degree of water crystalisation in a hydrated solid
Calculate mass of anhydrous solid then of water 2. Convert mass into moles 3. Anhydrous always =1 then divide water moles by the moles to give degree of hydration.
Equation for number of moles
Moles=mass divided by RFM