chemistry igcse edexcel
describe the structure of solids (4)
1) strong forces of attraction between particles = fixed lattice arrangement.
2) particles cannot move, but can vibrate about their positions.
3) heat causes them to vibrate more - solid expands slightly.
4) definite shape and volume
describe the structure of liquids (4)
> weak forces of attraction between particles = can move past each other, though they tend to stick closely together.
> particles are constantly moving w/ random motion.
> heat causes them to move faster - liquid expands slightly.
> definite volume, but no definite shape.
1/192
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
193 Terms
describe the structure of solids (4)
1) strong forces of attraction between particles = fixed lattice arrangement.
2) particles cannot move, but can vibrate about their positions.
3) heat causes them to vibrate more - solid expands slightly.
4) definite shape and volume
describe the structure of liquids (4)
> weak forces of attraction between particles = can move past each other, though they tend to stick closely together.
> particles are constantly moving w/ random motion.
> heat causes them to move faster - liquid expands slightly.
> definite volume, but no definite shape.
describe the structure of gases (4)
1) Extremely weak forces of attraction between particles = can move freely.
2) Particles are constantly moving w/ random motion.
3) Heat causes them to move faster - gas either expands slightly or increases in pressure.
4) No definite shape or volume
the state change of solid to gas
sublimation
describe the state change of solid to liquid to gas.
1. a solid is heated. its particles gain energy, causing them to vibrate about their positions and weaken the forces holding the solid together.
2. at a certain temp, the particles have enough energy to break free of the bonds that hold them in their positions.
3. the now-liquid is heated. its particles gain even more energy, causing the particles to move faster and weaken the bonds holding the liquid together.
4. at a certain temp, the particles have enough energy to break free of the bonds— turning into gas
briefly describe the diffusion practicals
1. place purple potassium permanganate at the bottom of a beaker of water and watch the colour spread out to fill the beaker.
2. soak cotton wool in aqueous ammonia and place it on one end of a horizontal glass tube. on the other, place cotton wool soaked in hydrochloric acid. a white ring of ammonium chloride forms between them, closer to the hydrochloric acid end due to lower mass of ammonia
3. fill half a gas jar with bromine gas and the other with air. separate w/ plate. remove plate - brown gas diffuses through air.
describe how dilution works
When water is added to a solution, causing particles to spread out— becoming less concentrated
what is solution?
mixture of solute and solvent
what is solute?
the substance being dissolved
what is solvent?
the liquid the solute dissolves into
what is a saturated solution?
in which the maximum amount of solute has been dissolved - no more solute will dissolve into the solution
what is an element?
consisting of only one type of atom.
what is a compound?
two or more elements chemically bonded together
what is a mixture?
two or more substances that are not chemically bonded - they can be separated.
what does filtration separate and how?
separates an insoluble solid from a liquid.
1. put filter paper into a funnel and pour mixture - liquid part runs through paper, leaving behind solid residue.
what does crystallisation separate and how?
separates a soluble solid from a solution.
1. pour solution into evaporating dish and gently heat - some water will evaporate and the solution becomes more concentrated.
2. once some water has evaporated, remove dish from heat and leave to cool.
3. salt should start to form crystals.
4. filter crystals out of solution and dry.
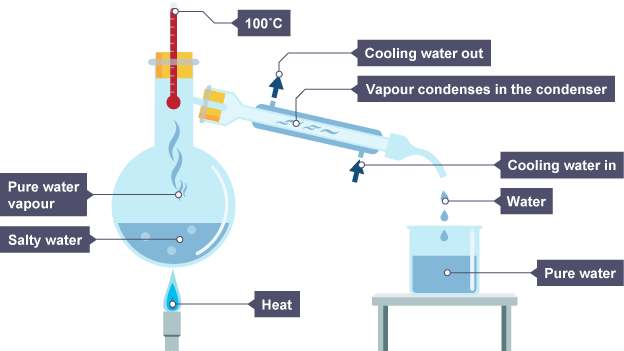
what does simple distillation separate and how?
Use: Separates liquid from solution (e.g. pure water from seawater).
Solution is heated, causing water vapour to rise & evaporate
Water vapour pass through condenser where it cools and condenses, turning into a liquid that is collected in a beaker
After all water is evaporated from solution, solute will be left behind
what does fractional distillation separate and how? (practical not real column)
separates mixture of liquids.
> the fractionating column is full of small glass rods, and is cooler at the top than it is at the bottom.
1. put mixture in flask and place column on top.
2. diff. liquids have diff. b.ps - heat flask to lowest b.p.
3. liquid evaporates, rising up column, and is then condensed. if other liquids evaporate, they make contact with the rods lower than their b.p. and condense, running back down.
describe the process of paper chromatography
1. draw a baseline w/ pencil (insoluble) near the bottom of a sheet of chromatography paper.
2. add spots of inks to the line at regular intervals.
3. roll sheet and place in beaker of solvent
4. ensure level of solvent is below baseline so ink doesn't dissolve.
5. place lid on container. the solvent seeps up the paper, carrying the ink with it. each dye moves at a different rate & forms a spot in a different place
6. when the solvent has nearly reached the top of the paper, take paper out and leave to dry = chromatogram
explain how chromatography works
1) Pencil line is drawn on chromatography paper and concentrated spot of ink/dye is placed on it
2) Paper is lowered into solvent, allowing solvent to travel up the paper carrying particles of coloured substance with it
3) Different substances have different solubilities so will travel at different rates, causing substance to be spread along vertical length of paper
→ this will show the different components of the ink/dye
equation for Rf value
distance travelled by solute / distance travelled by solvent
how can chromatography be used to identify a component of a mixture?
A pure sample of the substance runs alongside sample of the mixture
→ if sample has the same Rf value as one of the spots, they're likely to be the same
what does the distance a component travels represent on a chromatogram?
the distance a component travels on chromatogram indicates solubility in solvent
what is an atom?
the smallest particle of an element, containing protons, neutrons, and electrons.
what is a molecule?
a group of atoms joined together.
what is the atomic number?
number of protons
what is the mass number?
number of protons + neutrons
what is an isotope?
isotopes are different atomic forms of the same element - they have the same number of protons, but a diff number of neutrons
equation for relative atomic mass
Ar = (mass x abundance) + (mass x abundance) / sum of abundances (100)
what are the columns on the periodic table?
groups
what are the row on the periodic table?
periods
how can you use the periodic table to deduce electronic configuration?
the group number tell you the number of electrons in the outer shell. the period number tells you the number of shells there are around the atom.
how can you use the periodic table to know if something is a metal or non-metal?
non-metals on the right, metals on the left.
in terms of pH and conductivity, how can you tell if something is a metal?
Metals conduct electricity
Metal oxides are basic, neutralising acids
They dissolve to form solutions w/ a pH of more than 7
what do elements in the same group have in common and why?
they have similar chemical properties because they have the same number of electrons in their outer shell.
describe group 0 of the periodic table
Inert and colourless
→ full outer shell of electrons so do not react
equation for relative molecular mass
Mr = (Ar x no. of atoms) + (Ar x no. of atoms) …
equation for moles
moles = mass / mr
what is percentage yield?
How much product was made compared to the max. possible amount
equation for percentage yield
PY = (actual yield / max yield) x 100
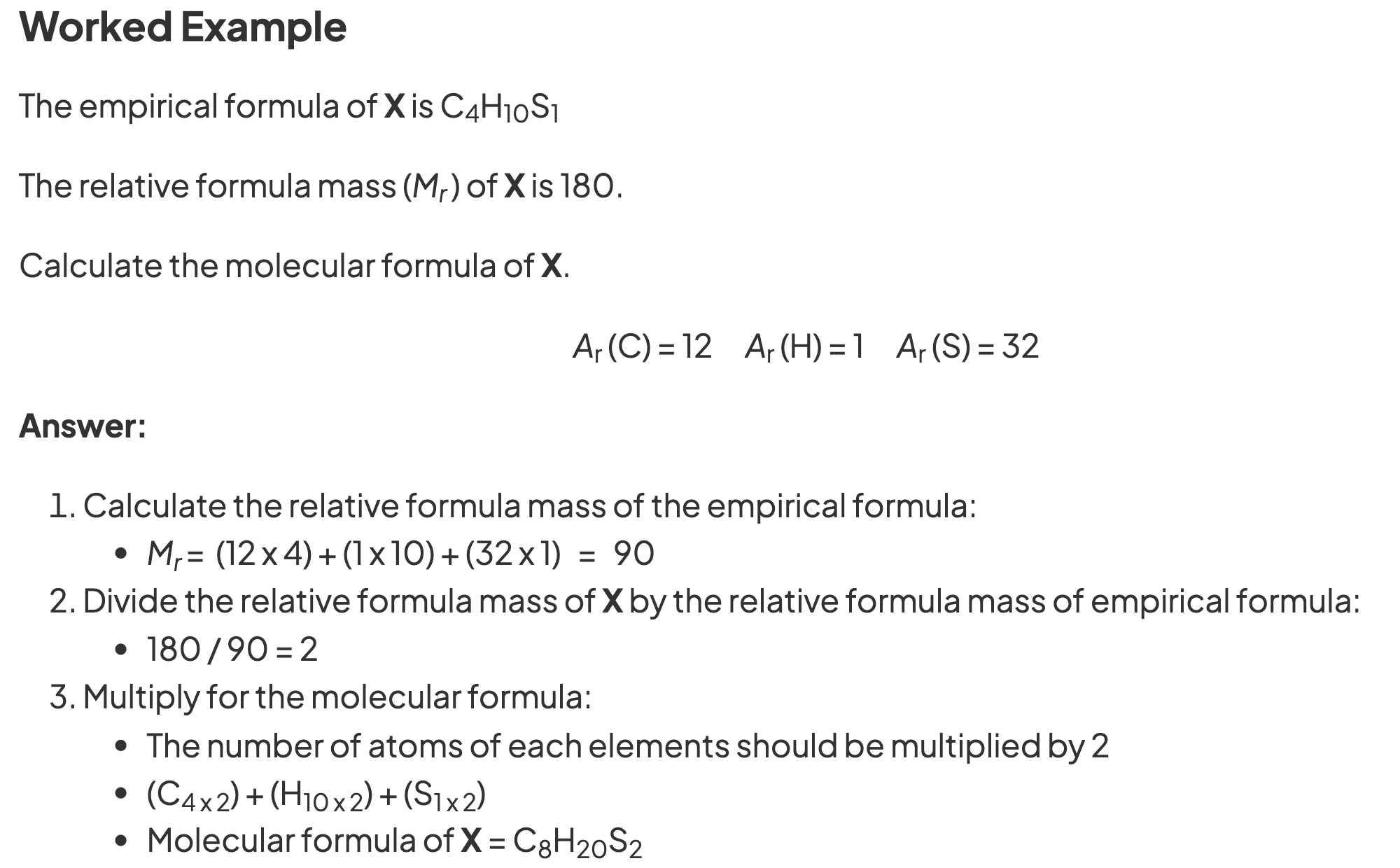
what are the steps to calculate empirical formula?
1. find moles of each element (using mass/Mr)
2. divide all moles by smallest no. of moles
what are the steps to calculate molecular formula?
find empirical formula
calculate Mr of empirical formula (multiply Ar of each element by no. of atoms)
divide given Mr (given in question) by empirical formula Mr
multiply empirical formula by this value
what is one decimetre in centimetres?
1dm = 10cm
practical - combustion of metal oxides using magnesium ribbon & crucible
Weigh empty crucible with lid.
Add magnesium ribbon, weigh crucible, lid + magnesium.
Calculate mass of magnesium (subtract empty crucible mass).
Heat crucible strongly with lid on, lifting lid occasionally to let air in but keep MgO inside.
Continue heating until mass stops changing (reaction complete).
Weigh crucible + contents.
Calculate final mass of magnesium oxide (subtract empty crucible mass).
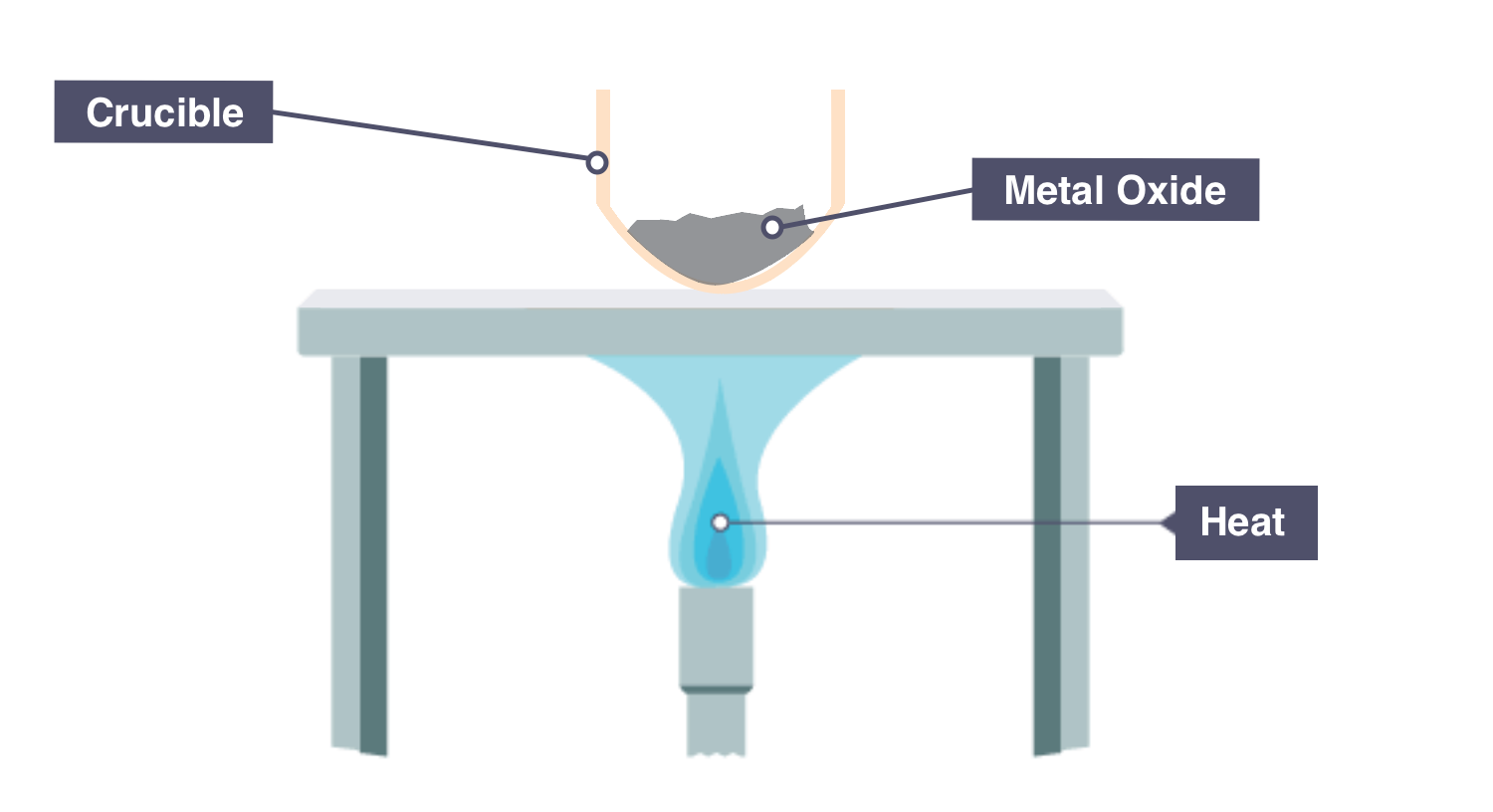
practical - how can you obtain formulae experimentally with reduction?
⚠ copper (II) oxide
Measure mass of Metal Oxide [copper (II) oxide]
Use a clamp to hold boiling tube horizontally, and place copper (II) oxide at the end of the tube
Heat using a bunsen burner until copper (II) oxide completely changed colour, indicating that all oxygen has been reduced
Measure mass of the remaining Metal powder
Empirical formula:
![<p><strong><span data-name="warning" data-type="emoji">⚠</span> copper (II) oxide</strong></p><ol><li><p>Measure mass of Metal Oxide [copper (II) oxide]</p></li><li><p>Use a clamp to hold boiling tube horizontally, and place copper (II) oxide at the end of the tube</p></li><li><p>Heat using a bunsen burner until copper (II) oxide completely changed colour, indicating that all oxygen has been reduced</p></li><li><p>Measure mass of the remaining Metal powder</p></li></ol><p></p><ol start="5"><li><p>Empirical formula:</p></li></ol><img src="https://knowt-user-attachments.s3.amazonaws.com/aae45303-12dc-4d50-b0b4-1377e3fd97f4.png" data-width="100%" data-align="center"><p></p>](https://knowt-user-attachments.s3.amazonaws.com/1ce70d18-b1b9-401f-9a40-0ef77b7790f3.png)
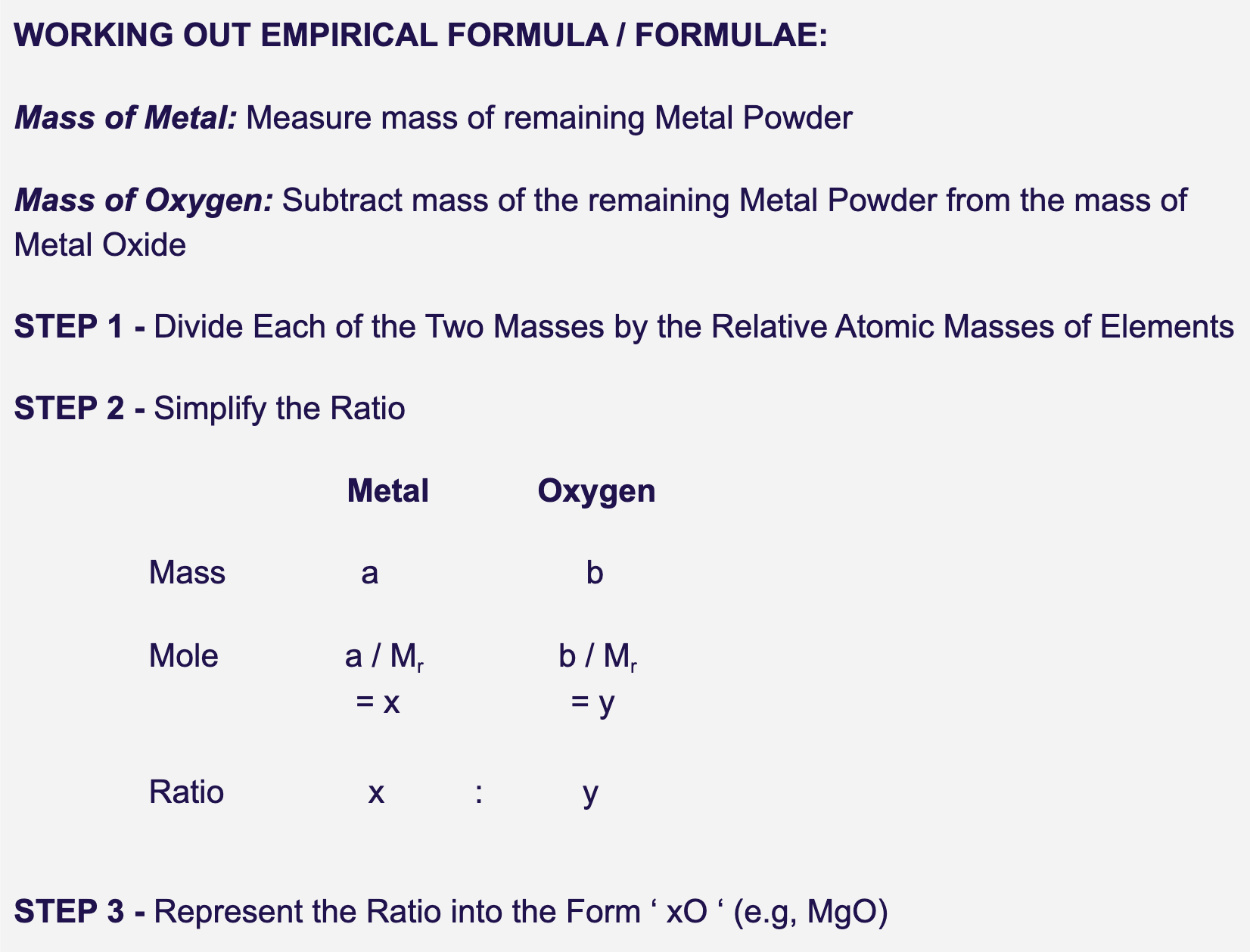
how can the info obtained from a combustion / reduction practical give the empirical formula of an oxide?
1. determine mass of each element (ex. Mg and O)
2. find moles of each of the elements (ex. Mg and O).
3. find simplest ratio by dividing by smallest no. moles.
how are ions formed?
when atoms gain or lose electrons.
what charge would groups 1, 2, and 3 metal ions have?
1+, 2+, and 3+
what charge would group 5 ions have?
3-
what is the formula for a hydroxide ion (with charge)?
(OH)-
what is the formula for a copper ion (with charge)?
Cu2+
what is the formula for a carbonate ion (with charge)?
(CO3)2-
what is the formula for an iron ion (with charge)?
Fe(ii)2+ or Fe(iii)3+
what is the formula for an ammonium ion (with charge)?
(NH4)+
what is the formula for a lead ion (with charge)?
Pb2+
what is the formula for a sulfate ion (with charge)?
(SO4)2-
what is the formula for a hydrogen ion (with charge)?
H+
what is the formula for a nitrate ion (with charge)?
(NO3)-
what is the formula for a silver ion (with charge)?
Ag+
what is the formula for a ammonium ion (with charge)?
(NH4)+
what is the formula for a zinc ion (with charge)?
Zn2+
what happens in ionic bonding?
when a metal and non-metal react together and the metal atom loses electrons to form a cation and the non-metal gains these electrons to form an anion.
what is an ionic bond?
the electrostatic attraction between positively charged metal and negatively charged non-metal ion
what must be remembered when working out the formula of an ionic compound?
> the overall charge must be zero.
> negative charges must balance out positives.
> you can just flip the charges!
describe a giant ionic lattice structure (including boiling point & conductivity).
> ions are held together in a closely-packed lattice arrangement by the attraction of oppositely charged ions.
> electrostatic attraction = strong. a lot of energy is needed to overcome strong attraction.
> high boiling point.
> conducts electricity when molten or dissolved in aq. solution.
what happens in covalent bonding?
when non-metal atoms share electrons to obtain a full outer shell.
what is a covalent bond?
the electrostatic attraction between a shared pair of negatively charged electrons and the positively charged nuclei of atoms involved.
what are the different covalent structures?
giant covalent lattice & simple molecular
describe a giant covalent lattice structure (5)
1) All atoms are bonded & held by covalent bonds
2) Atoms are in a closely-packed lattice arrangement
3) Strong bonds, so a lot of energy needed to overcome them
4) High boiling point
5) Doesn't conduct electricity - except for graphite
describe the structure of a diamond and its properties (5)
1) Giant covalent lattice
2) Carbon atoms each form four covalent bonds
3) Strong covalent bonds hold atoms in rigid lattice structure = very hard
4) A lot of energy needed to break strong covalent bonds - high boiling point & melting point
5) No free electrons or ions - does not conduct electricity
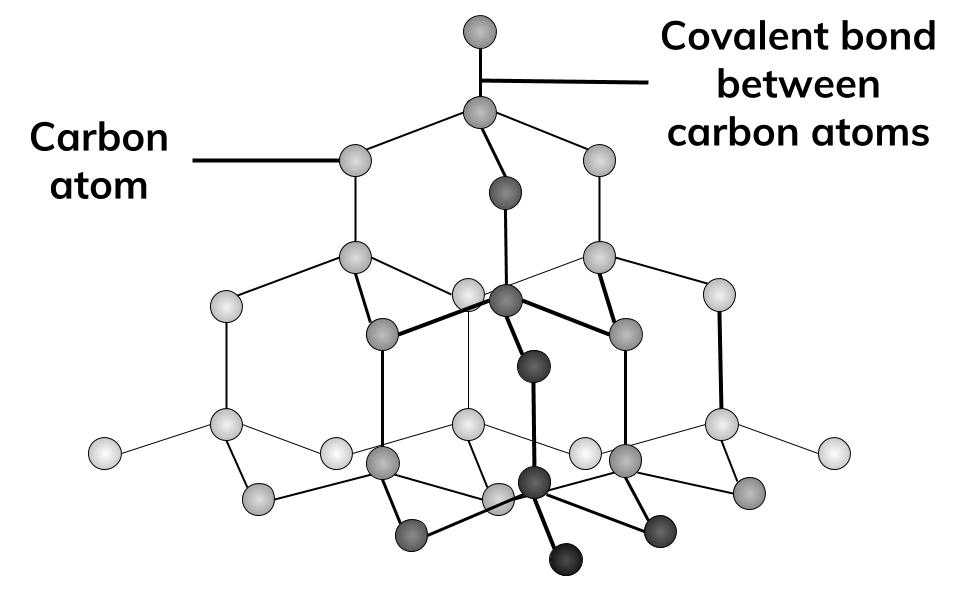
describe the structure of graphite and its properties. (5)
1) Giant covalent lattice
2) Each carbon atom forms only three covalent bonds, creating layers of carbon atoms.
3) Layers are held by weak intermolecular forces and are able to slide over each other - slippery
4) Covalent bonds in layers need a lot of energy to break - high boiling point
5) Only 3/4 used so each carbon atom has 1 delocalised electron → conducts electricity
describe the structure of C60 fullerene and its properties (4)
1) Simple molecular structure with strong covalent bonds
2) 60 carbon atoms held by weak intermolecular forces so molecules can slide over each other = soft
3) Less energy needed to overcome forces = low boiling point
4) 1 delocalised electron per carbon, however electrons can't move between molecules = poor conductor
describe a simple molecular substance structure (including boiling point and conductivity). (4)
1) Atoms within each molecule are held together by strong covalent bonds.
2) However, intermolecular forces are weak.
3) Low bp.
4) Most are gases or liquids at room temp due to low bp.
why do boiling points of simple molecular substances increase with increasing relative mass?
if they have a higher relative mass, there are more points along the larger molecule for intermolecular forces to act.
why do covalent compounds not conduct electricity?
they don’t contain any freely moving charged particles to carry charge.
why do ionic compounds conduct electricity only when molten or in aqueous solution?
ionic compounds contain charged ions, but when they are solids, the ions are held in a fixed lattice structure. when molten or in aq. solution, the ions are free to move.
what happens at the cathode?
cations are attracted to it and gain electrons (reduced) to become atoms.
what is group 1 of the periodic table called?
Alkali metals
what is the equation of an alkali metals' reaction with water?
(metal) + water --> (metal) hydroxide + hydrogen
what are the products of the alkali metals' (lithium, sodium, and potassium) reactions with oxygen in the air?
they react with oxygen to form metal oxides.
> lithium reacts to form lithium oxide.
> sodium and potassium react to form different types of oxides (peroxides, superoxides).
describe the qualitative observations of the reaction of group 1 metals with water. (4)
1) fizzing
2) melts into shiny ball moving around the surface.
3) disappearing
4) potassium burns with a lilac flame
what is group 7 of the periodic table called?
halogens
what are the colours and physical states of group 7 elements (chlorine, bromine, and iodine)?
chlorine is a green gas.
bromine is a red-brown liquid.
iodine is a dark grey solid.
how can you use knowledge of trends in group 7 to predict the properties of other halogens?
as you go down group 7, halogens become...
> less reactive.
> darker in colour.
> higher in melting / boiling point.
what is displacement?
when a more reactive element "replaces" a less reactive element from a compound.
how do displacement reactions link to redox?
displacement reactions always involve the transfer of electrons - the more reactive element is oxidised (loses electrons) and the less reactive element is reduced (gains electrons).
what is an oxidising agent?
accepts electrons and gets reduced.
what is a reducing agent?
donates electrons and gets oxidised.
in terms of displacement reactions, which element is the oxidising agent?
the element that is being displaced. it accepts electrons and is reduced.
what are the percentages of gases in the atmosphere?
nitrogen - 78%
oxygen - 21%
argon - 0.9%
carbon dioxide - 0.04%
describe the practical for investigating volume of oxygen in the air using a metal (iron)
⚠ using iron
Place wet Iron fillings at the end of a burette
Use a clamp to hold the burette invertedly in the trough of Water
Measure and note the starting height of Water level in the burette
Leave apparatus for several days
Measure and note the final height of Water level in the burette
Calculate % of O2:

Volume of Oxygen Used = (Initial Vol. of Air) - (Burette - Final Vol. of Water)
Volume of Air at the Start = ( Burette Volume - Initial Reading)
what is the equation for the percentage of oxygen in the air?
% of O2 = (start vol - final vol) / start vol x 100
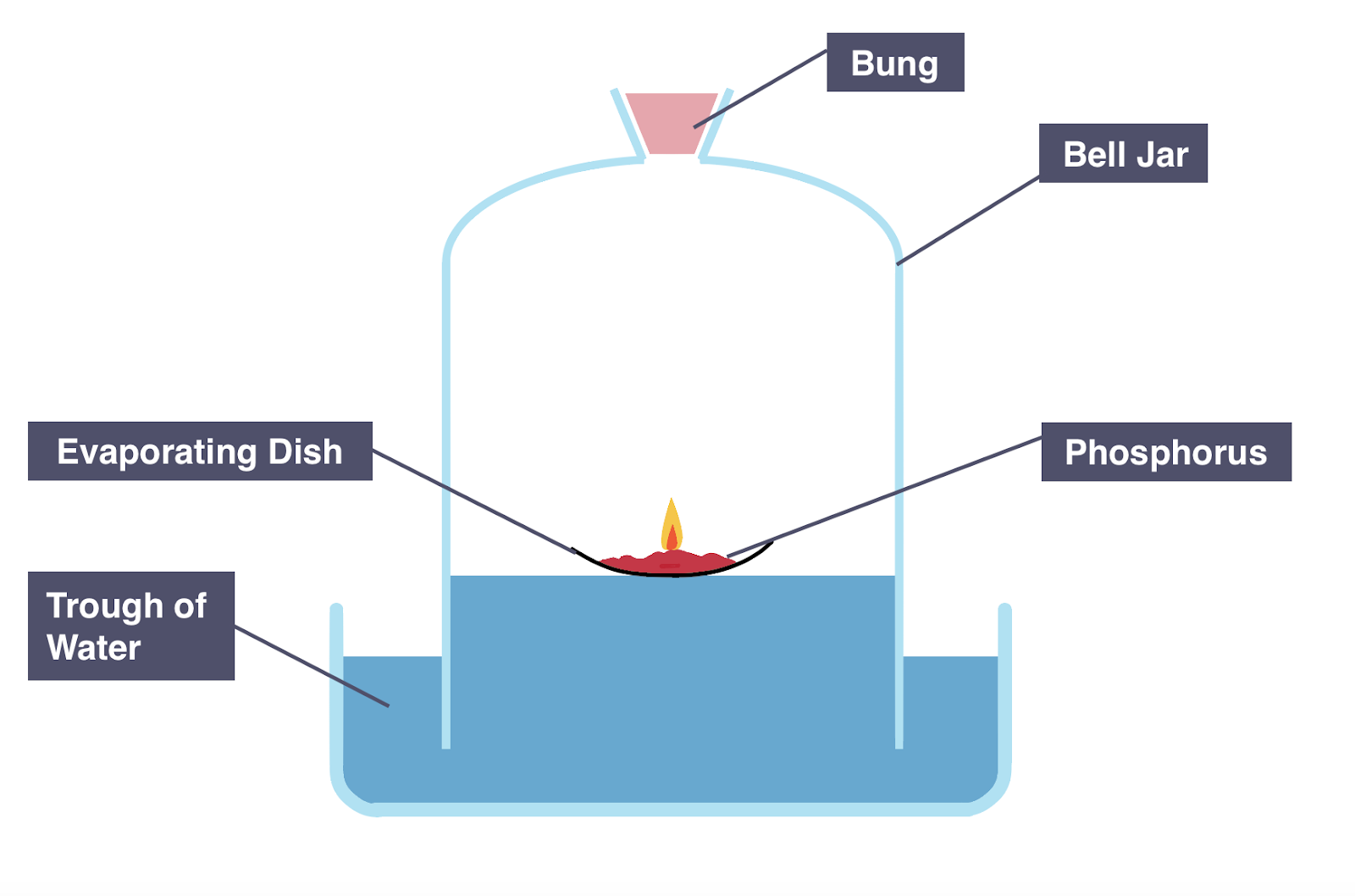
describe the practical for investigating volume of oxygen in the air using a non-metal.
⚠ use phosphorus!
Add phosphorus into an evaporating dish and place it on a trough of Water
Ignite Phosphorus using a candle
Cover evaporating dish with a bell jar
Measure & note the starting height of the Water level in the bell jar
Leave apparatus for several days
Measure & note the final height of the Water level in the bell jar
what is combustion?
burning something so that it reacts with oxygen in the air.
describe the flame colour and products of the combustion of magnesium, hydrogen, and sulphur.
Magnesium - burns with a bright white flame and forms white powder (magnesium oxide).
Hydrogen - burns with an almost invisible pale blue flame and produces water.
Sulphur - burns with a pale blue flame and produces sulphur dioxide.
is sulphur dioxide acidic or alkaline when dissolved in water?
acidic.
why is the combustion of hydrogen dangerous?
it is explosive and flammable. think molotov cocktail
what is the equation for thermal decomposition of a metal carbonate?
(metal) carbonate + oxygen --> (metal) oxide + carbon dioxide
describe the thermal decomposition of copper(ii) carbonate.
Copper(ii) carbonate is a green powder.
When it is heated, it forms CO2 and copper(ii) oxide (black)
how does carbon dioxide in the atmosphere contribute to climate change?
it is a greenhouse gas. greenhouse gases absorb most of the heat that would normally be radiated out into space and re-radiates it in all directions, including back towards earth.