PHAR 307 - Lecture 13: mass spectrum interpretation
1/20
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
21 Terms
molecular ion peak
ion formed after undergoing ionization and not fragmenting, usually last peak but sometimes missing
molecular adducts
ions formed when molecule interacts with other species such as solvent molecules, counterions, small ions
isotopic peaks
molecular ion peaks with smaller mass unit peaks due to presence of different isotopic masses of element
energy needed to remove electron from highest to lowest
sigma bonds, non conj pi bonds, conj pi bonds, non bonding or lone pair
order of stability highest to lowest
aromatic, alicyclic, n-hydrocarbons, ketone, branched chain hydrocarbon, alcohols
daughter ions are often
cations and radicals
fragmentation of alkanes
occurs adjacent to most substituted carbon atom
fragmentation of alkenes
allylic carbocations favored
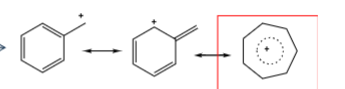
fragmentation of aromatics
benzene and phenyl = most stable species = will not fragment further, if possible species will form tropylium ion
tropylium ion formation
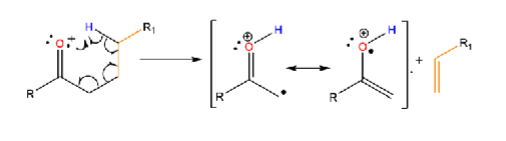
alpha cleavage
formation of double bond due to fragmentation (electron from ionized atom + one from sigma bond)
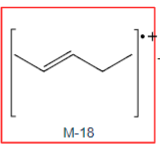
loss of water
M-18
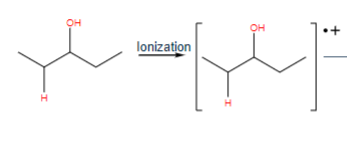
what is the product (loss of water)
+H2O
alpha cleavage of alkyl radical cleaves
preferentially largest group
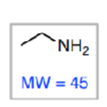
preferentially fragments into
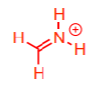
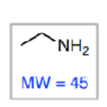
less preferably fragments into
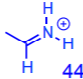
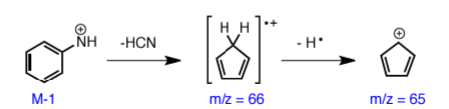
anilines commonly fragment off
HCN
aniline fragmentation
McLafferty rearrangment
transfer of hydrogen from one part of molecular ion to another
McLafferty rearrangement conditions
appropriately located heteroatom (non H or C), double bond, extractable hydrogen atom which is gamma to C=O system
McLafferty rearrangement process