alevel biology structure
1/15
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
16 Terms
Enzyme
Biological Catalyst which lowers activation energy by providing an alternate reaction pathway without being used up.
Globular protein with a depression in structure giving active site
Substrate in enzyme holds substance closer together, so easier to form bonds by minimising repulsion
Shape can strain bonds and allow molecule to be more easily broken
Induced fit
Lock and Key
Substrate binds to the active site of enzyme,
active site changes shape to be complementary to the enzyme
Enzyme active site is complementary to the substrate, and only the substrate can fit in the active site
Enzyme full functioning
Stress bonds to make/break polymers
Substrate in enzyme holds substance closer together, so easier to form bonds by minimising repulsion
Shape can strain bonds and allow molecule to be more easily broken
DNA
RNA
DNA and RNA are used to store genetic information in a cell
Consists of a double helix with two strands in antiparallel. With a pentose-phosphate backbone. strand goes from 3 prime end —> 5 prime end
RNA is shorter, single strand for translation, also has ribose sugars not deoxyribose.
DNA | RNA |
Deoxyribose | Ribose |
A-T, C-G | A-U, C-G |
Double helix/ strand | Single stranded |
Long | Short |
Sugar-phosphate backbone | Sugar phosphate backbone |
DNA structure
How structure of dna helps with function
Polynucleotide joined together with phosphodiester bonds between phosphate of one nucleotide and deoxyribose of another nucleotide via a condensation reaciton
Nucleotides consist of a: nitrogenous base bonded to a pentose sugar, and a phosphate.
2 strands - both can act as templates for semi-conservative replication, hence saving energy
H-bonds → easily separated for replication
Many h-bonds → stable molecule
Sugar-phosphate backbone - protects h-bonds
Double helix - compact
ATP
Adenosine triphosphate.
ribose bonded to adenine, and 3 phosphates, via a condensation reaction between the adenosine, phosphate and ribose with ATP synthase.
Uses which make it suitable:
Inorganic phosphate is used for phosphorylation to produce more reactive substances
Little energy released as heat
Easily resynthesized
Energy can be used for metabolism, contraction, active transport, secretion, and molecule activation
Unstable bonds so releases energy instantly
cannot be transported out of cell
Semi-conservative replication
DNA helicase unwinds double helix, to form a dna fork, where both strands act as template for a new one
DNA primase attaches RNA primer to dna
Free floating nucleotides form hydrogen bonds to dna Bases via complementary base pairing
DNA polymerase III bonds the nucleotides of new strand by forming phosphodiester bonds.
Antiparallel causes one strand to be made continuously, whilst the other is made in okazaki fragments. with continuous primers being added, with bases bonded together
DNA exonuclease removes primers, polymerase I fills in gaps
DNA ligase joins the strands together
Enzyme Inhbition
Inhibitor - substance that interferes with the functioning of an active site, directly or indirectly.
Inhibition is required to control reactions by stopping specific enzymes in a reaction.
End point inhibition - final molecule of enzyme reactions series, inhibits the first enzyme, maintaining steady concentration
Non-competitive:
Bind to allosteric site, which changes the tertiary structure of the enzyme, causing denaturing of the active site, and active site is no longer complementary to substrate
Competitive:
Similar structure to substrate, so directly binds to active site, which prevents substrates from binding, therefore temporarily inhibiting the enzyme
Factor Affecting enzyme controlled reactions
1.Temperature
2. Ph
3. Enzyme Concentration
4. Substrate Concentration
More kinetic energy so substrate and enzyme collide more frequently, forming more enzyme-substrate complexes, increasing rate of reaction. Too high causes hydrogen bonds in chain to be broken losing active site shape.
Too high denatures, too many H+ ions effecting bonding, altering amino acids
More enzymes for substrate to collide with, so higher chance of enzyme substrate complex. More active sites to bind to.
More substrates to bind to enzymes, however reaches plateau as all enzymes have active site in use
Water (properties which make it useful), M,S,HL,HS,CO,LV,TE,AD
metabolite in respiration
solvent, metabollic reactions can take faster place within water
high latent heat of vaporisation, so cools organism upon evaporation
high specific heat capacity, can buffer temperature changes
cohesive, suitable for plant transport as can support water columns
low viscosity, flows easily through narrow
handles tension, so force can be applied
adhesive, so high surface tension, when ice forms can resist changes to environment below
Monosaccharides, disaccharides. (polysaccharide definition, bonding type, carbons bonded + effect, disaccharides to learn)
long chain made up of small repeating monosaccharide units
Disaccharides have either 1-4 bonds or 1-6 bonds
1-4 produces straight chain
1-6 produces branching
maltose (reducing)= 2 a-glucose
lactose (reducing)= a-glucose + galactose
sucrose (non-reducing) = a-glucose + fructose
cellulose = b-glucose + b-glucose

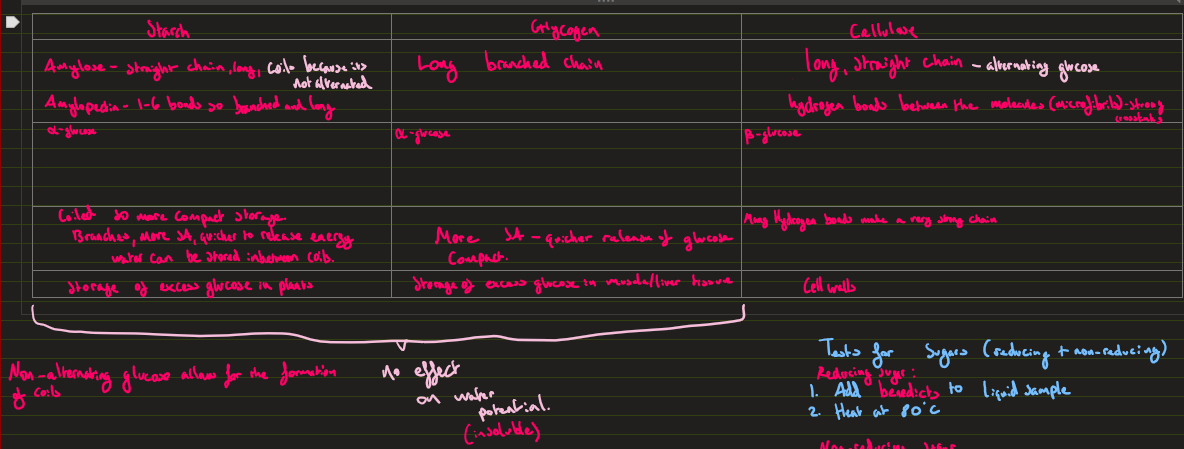
polysaccharides/ carbohydrates (starch, glycogen, cellulose) (properties, structure, uses)
Starch: storage of excess glucose in plants
Monomers: a-glucose, Amylose (straight chain, coils) Amylopectin ( 1-6 bonds, so branched and long)
Coiled so more compact storage, Branched so has a higher surface area, quicker to release energy, water can be stored inbetween coils
Glycogen: short term storage of excess glucose in animal
Long Branched chain of a-glucose
Larger sa, quicker release of energy,
Cellulose: cell walls of plants, B-glucose
Long straight chain of alternating glucose, microfibrils between chains
Very strong, resistant to excessive bending.
Testing for sugars
Reducing:
Add benedicts to liquid sample, Apply heat
Non-reducing:
Add benedicts, heat, no positive results = add dilute hcl, then neutralise with an alkali, add benedicts, heat.
Iodine: Presence of starch, brown —> blue-black
Lipids
Lipid: Biological molecules which are only soluble in organic solutions, used for long term energy storage, structure, insulation, protection
saturated = c-c bonds, unsaturated = c=c bonds
triglycerides
Glycerol + 3 fatty acids
Hydrophobic fatty acids, non-polar
phospholipids
Glycerol + phosphate + fatty acids
polar one end. Hydrophilic properties, means phosphate forces arrangement of fatty acids pointing inwards.
Proteins
Peptide bond - condensation reaction between amine group, and carboxyl groups of 2 amino acids, producing a water molecule.
Polypeptide, made up of multiple amino acids.
Protein can be made from multiple polypeptides
Primary structure: Sequence of bonded amino acids
Secondary structure: Amino acid chain bends into alpha helices, beta pleated sheets, as Hydrogen bonds form between charged hydrogens and charged Nitrogen, Oxygen
Tertiary structure: Twisting, and bending of chain,
additional hydrogen bonds, disulfide bridges between R groups, Ionic bonds, All between any unbonded charged atoms
Quaternary structure: Multipe polypeptide chains in a protein which work towards a function.
Chemical tests
Proteins:
Biuret, changes from blue-black to purple
Lipids:
Add ethanol, then water and shake, forms a white emulsion
Starch:
Add iodine blue-black colour change
Reducing sugar:
Add benedict’s and heat, forms red/orange/green colour precipitate