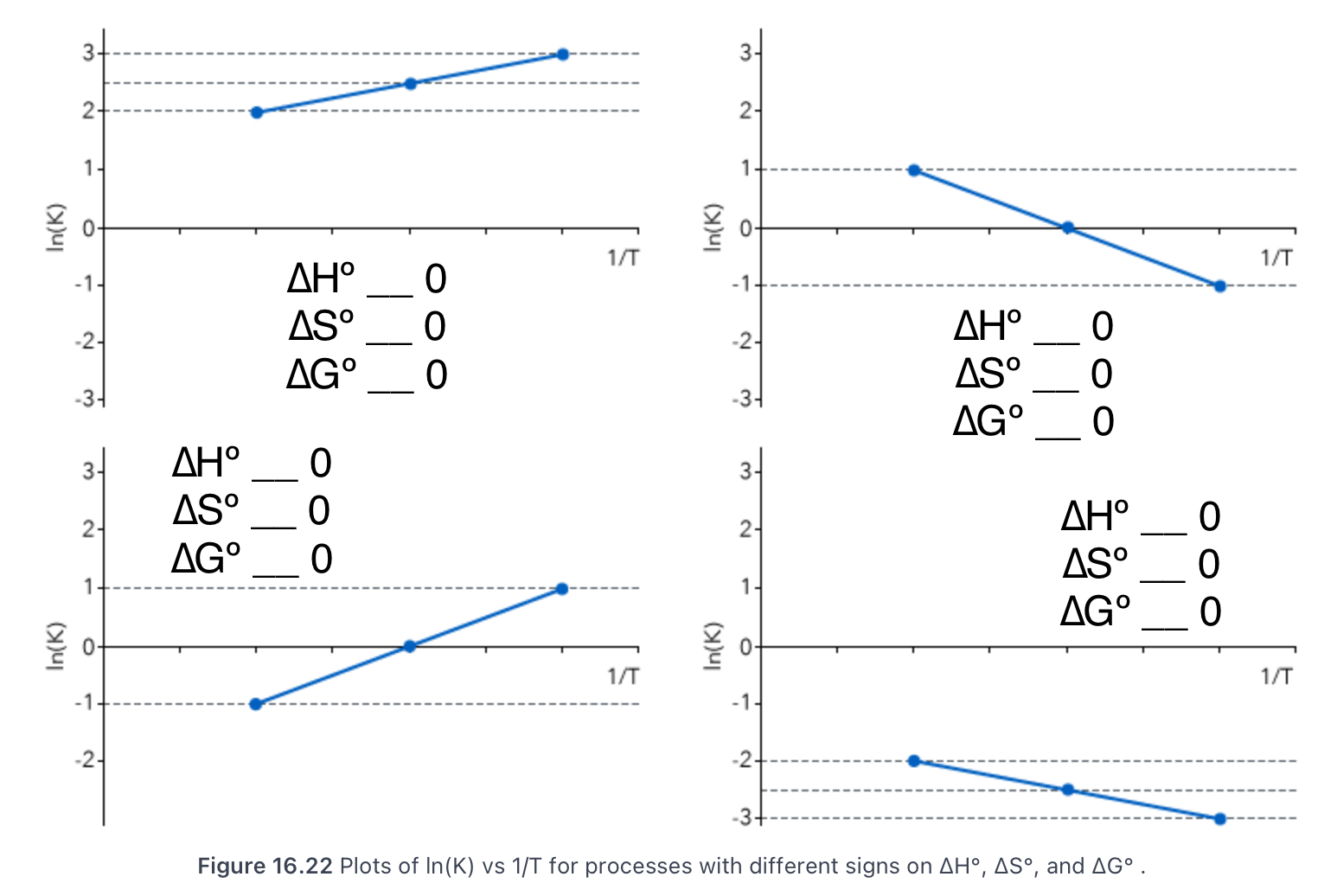
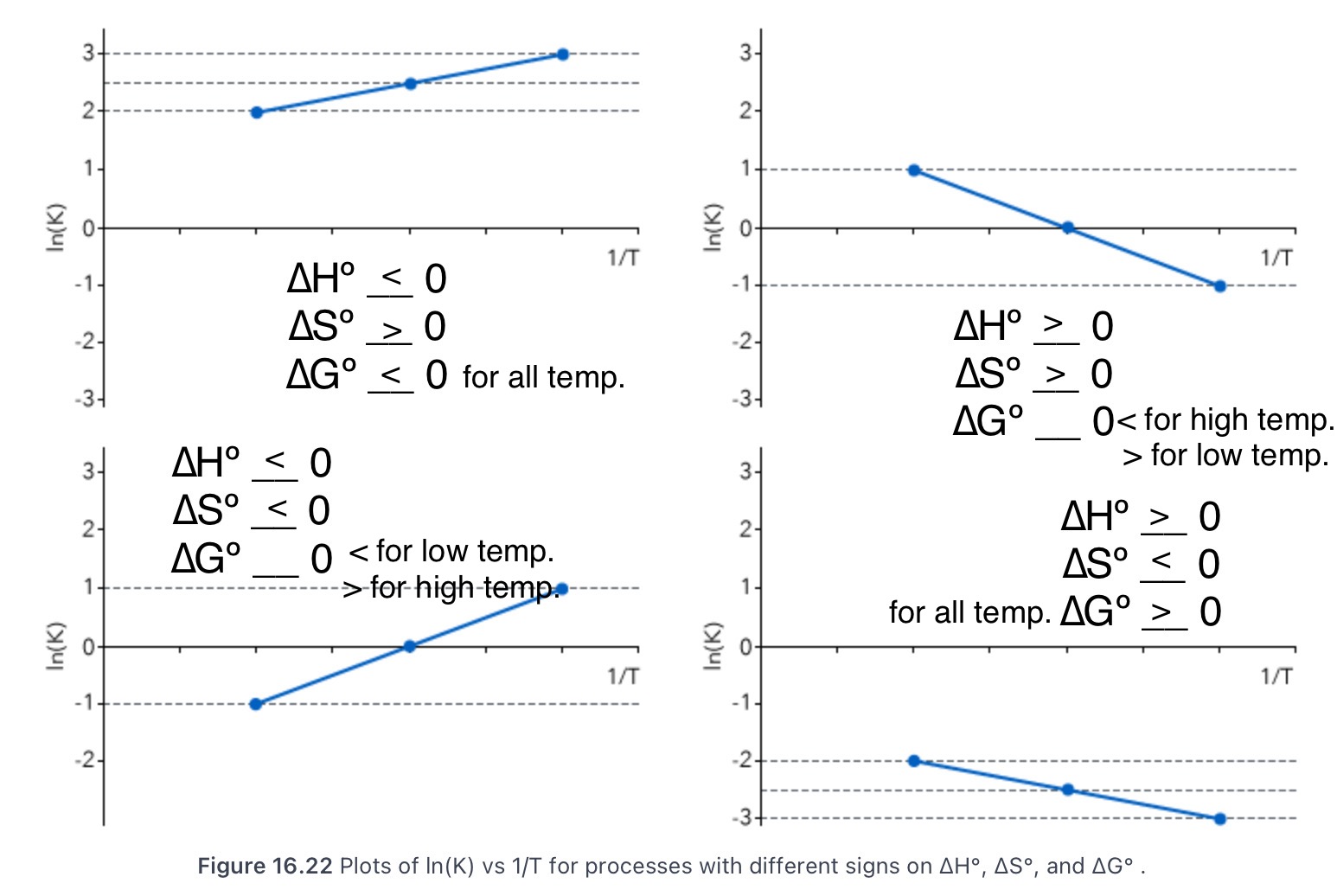
16.6 Temperature Dependence of the Equilibrium Constant
0.0(0)
Card Sorting
1/3
There's no tags or description
Looks like no tags are added yet.
Last updated 8:19 PM on 4/6/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
4 Terms
1
New cards
State the Clausius-Clapeyron equation and what it’s a special case of.
ln(K1 / K2) = -∆H/R (1 / T2 - 1 / T1); special case of the van’t Hoff equation for vapor/liquid equilibrium
2
New cards
State the van’t Hoff equation.
ln(K) = (-∆Hºrxn / R)(1 / T) + ∆Sºrxn / R
3
New cards
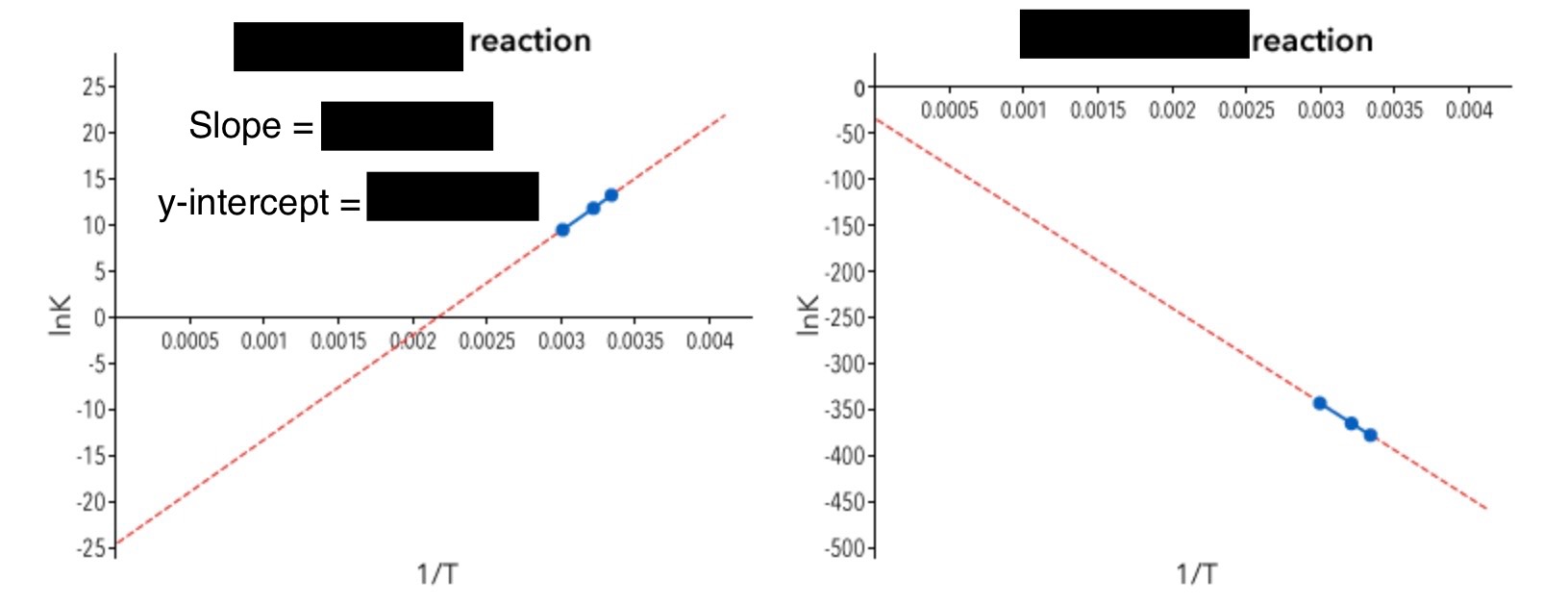
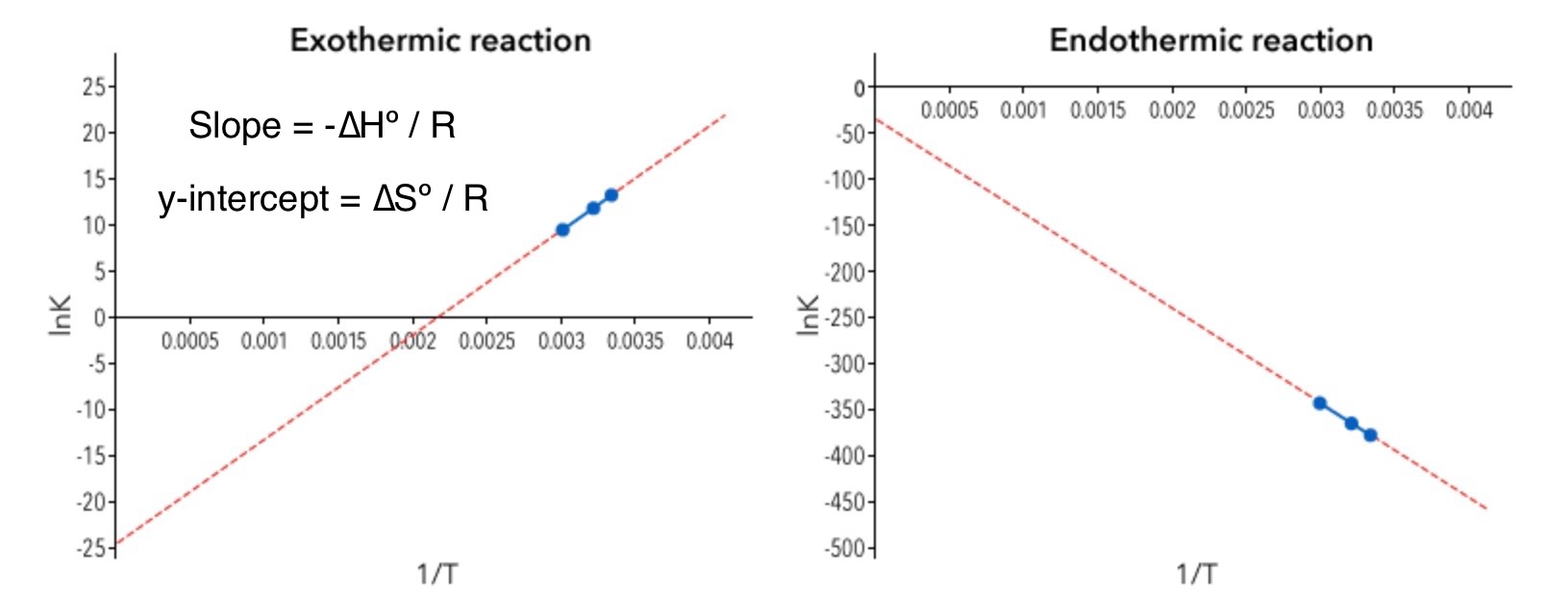
4
New cards