Common Mistakes AP CHEM
1/26
Earn XP
Description and Tags
for silly mistakes often made
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
A lower molar mass= more mols
Which means more pressure.
So equal masses of different gases will mean the lower molar mass substance will have higher partial pressure

Strength of bonds does not mean strength of IMF’s
While F2 has a smaller ionic radius which means its bonds are stronger, in terms of IMF’s the strength of intramolecular forces DOES NOT MATTER
Since they’re both nonpolar, and LDF’s are the only IMF’s present, Br2 has the higher melting point

The differences between 1st and 2nd order:
1st order doesn’t mean constant subtraction(like -10 each time) but constant percentages
Difference in electronegativity means more ionic character. The bigger the difference is the better
The farther apart elements are on the periodic table the better
Bond length does NOT relate to molecule size
*Any bond order or bond length question. Draw them out, you’ll never know until you do.
In this case, you while the O2 atom does have smaller atomic radius making the molecule smaller…
The N2 molecule has a triple bond(higher bond energy and shorter length) while O2 has a double
You would know this if you drew it out.
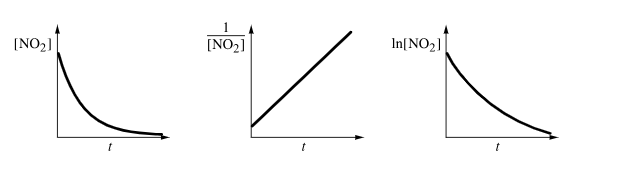
2nd order reaction
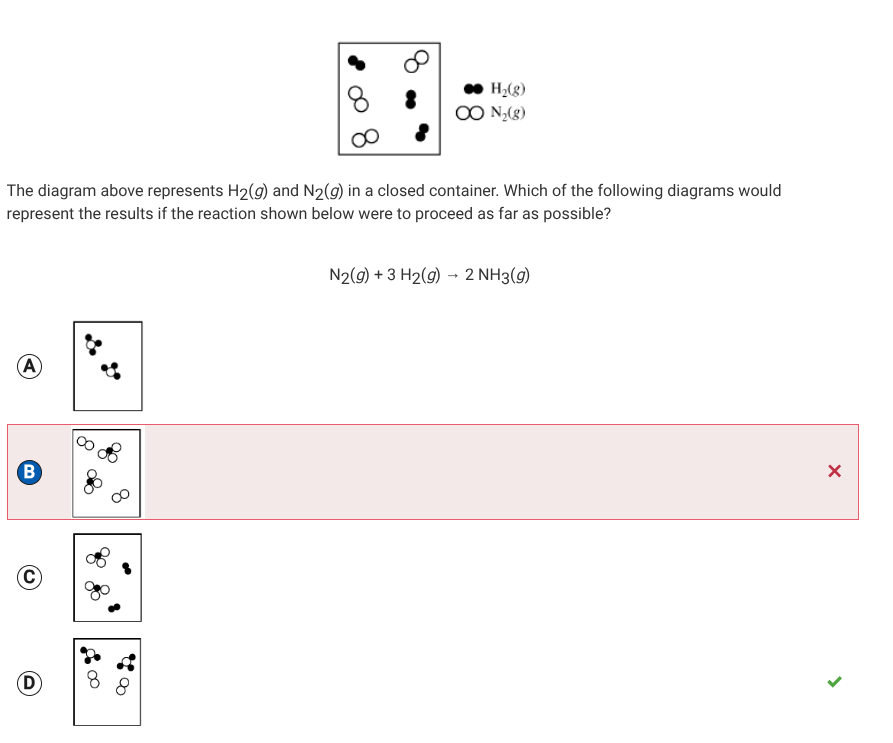
Make sure you identify the particles(you understand the concept, just make sure you’re doing the right things to prevent careless mistakes)
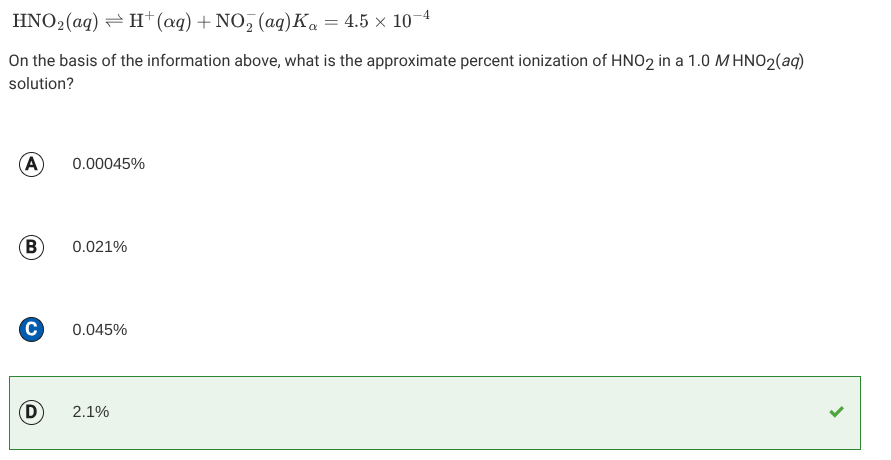
Ka values
Ka values are the acid dissociation constant, not % ionization.
Here, you square root the Ka values(x²) to find x, which is the concentration of the ionized, giving you % ionization
Always double check your algebra
Remember the time you made the PV=NRT equation wrong
yes sir!
Always double check your calculations
Sometimes you might have typed it wrong on the calc
yes sir!
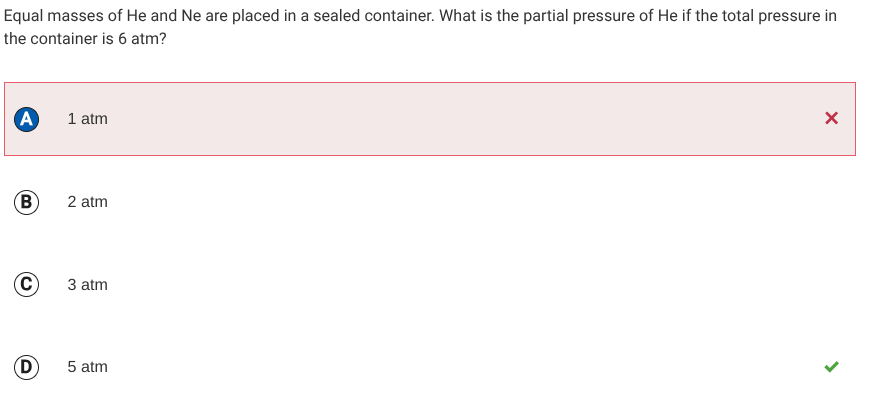
AGAIN, in this question
A lower molar mass would lead to an increase in # of moles of that molecules which means a higher partial pressure
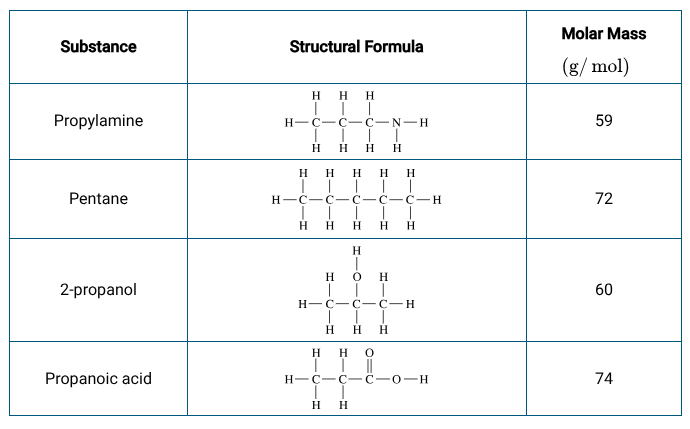
Why or why doesn’t 2-propanol disassociate in ions?
Only SNAP ions and strong acids/bases will lead to dissociation of ions in water
2-propanol doesn’t have a SNAP ions and isn’t a known strong acid/base so it SHOULD not dissociate into ions and any statement with that as its justification is wrong

Answer is greater than 7, the KOH is a base, basic solutions have a high pH
What should you always do in conversion of mols in differing parts of a reaction when the net ionic equation isn’t given?
ALWAYS find the mol ratios from balanced equation
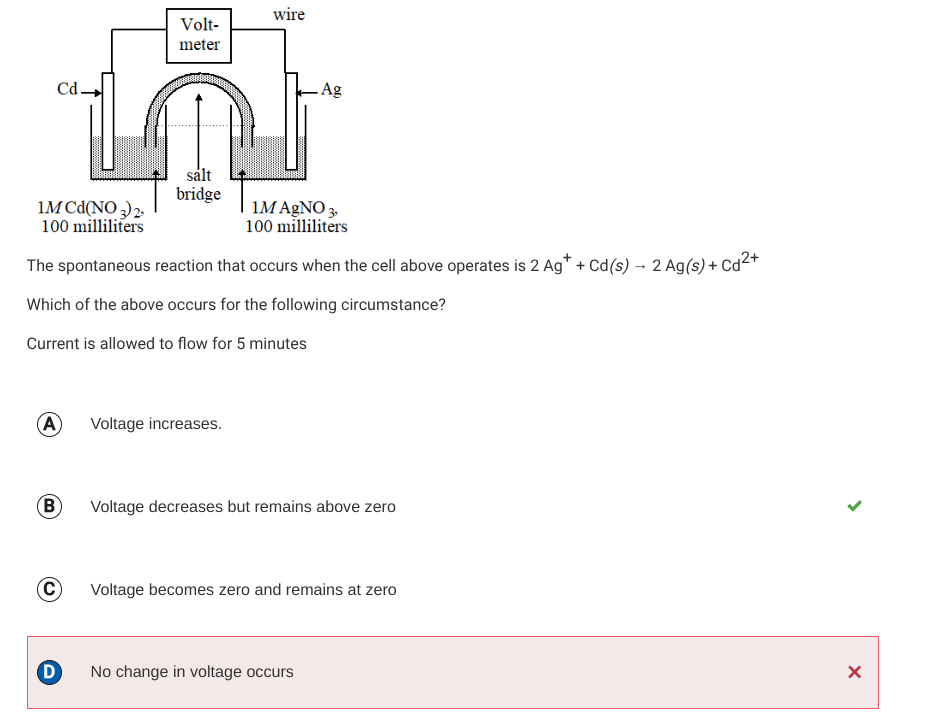
As negative delta G means a positive E cell, meaning that as the reaction occurs, and a galvanic cell is in operation, the delta G is negative(thermodynamically favored) and the reaction is ACTUALLY happening
Which mean… →
The reactants are being used up and products are being created.
Since there is literally less reactant(or battery) to react, the voltage decreases
Also *E cell and Voltage mean the same thing

Molar mass of Ca: 40g/mol
Solve it
75% because 30/40=3/4=75% was in the sample
Connect your answer to what the question is asking:
For example: if they ask for moles of Cl, what do you provide?
REMEMBER, it may seem silly, but when you calculate the mass of Cl, it is easy to forget to convert into moles
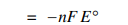
When calculating delta G with the Delta G=
Make sure to what?
Check for thermodynamically favorability and use the appropriate sign
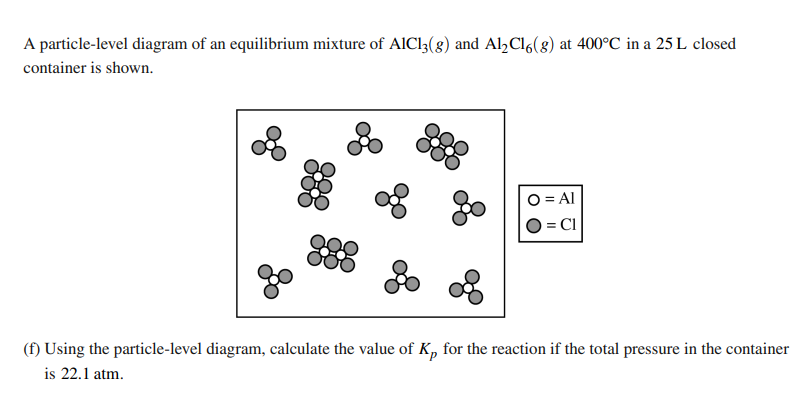
Solve this: with the Kp equation given with the equation
2AlCl3 ←→Al2Cl6
Find Kp
Make sure to use the correct ratios and the partial pressures with the correct conversion
What does a larger surface area/smaller particle size do to the rate of reaction and why?
Increases the rate of reaction, causing the reaction time to decrease because the frequency of SUCCESSFUL collisions increases
“this allows more reactant particles to collide with each other, enhancing the likelihood of effective interactions. “
Do you ever assume you know the atomic mass of a substance?
NO! I don’t trust you enough unless its O
Where do Ion-Dipole interactions occur?
Do they occur in say liquid solutions of HBr and HF
Where already dissasociated ions are present
So liquid HBR and HF do not disassociate so there are no ions present
Difference between Ionize and Disassociate?
Use disassociate when talking about a existing ionic compounds like NaCl
Use ionize with acids and bases
When they give you a particulate representation of the
IONS present in a equilibrium expression what do you not use to justify as an error in their diagram
Stating that since its an equilibrium expression, the particles should not be completely ionized and there should still be some solid
This is wrong because its just talking about the ions in the reaction, of course there isn’t a solid
Delta G is 0 at equilibrium
What is the only factor that changes the equilibrium expression k
Temperature, nothing else will change it
When they ask for the process that occurs in a weak base and strong acid solution…
Write ONLY what's reacting, the undissolved weak base won’t react, only its partially dissolved part WILL, so that's what you include.