Nitrogen
1/32
Earn XP
Description and Tags
Chapter 3 in the Lecture Manual
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
33 Terms
Define Nitrogen Cycle
the association of all interactive chemical and biochemical processes related to Nitrogen availability
Define Immobilization
the temporary assimilation of plant available soil Nitrogen into the organic systems of microbes
Define Mineralization
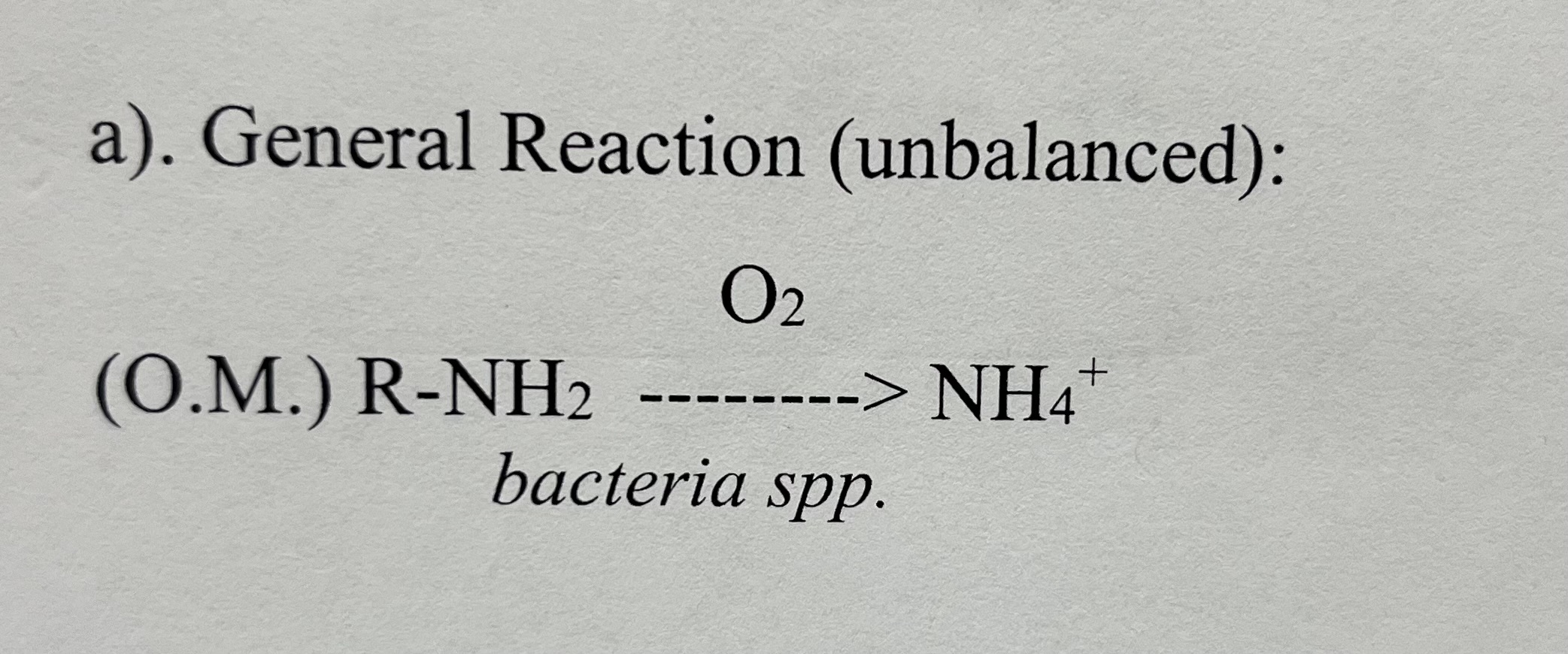
the transformation of organic soil Nitrogen forms to plant usable Nitrogen forms by microbial oxidation
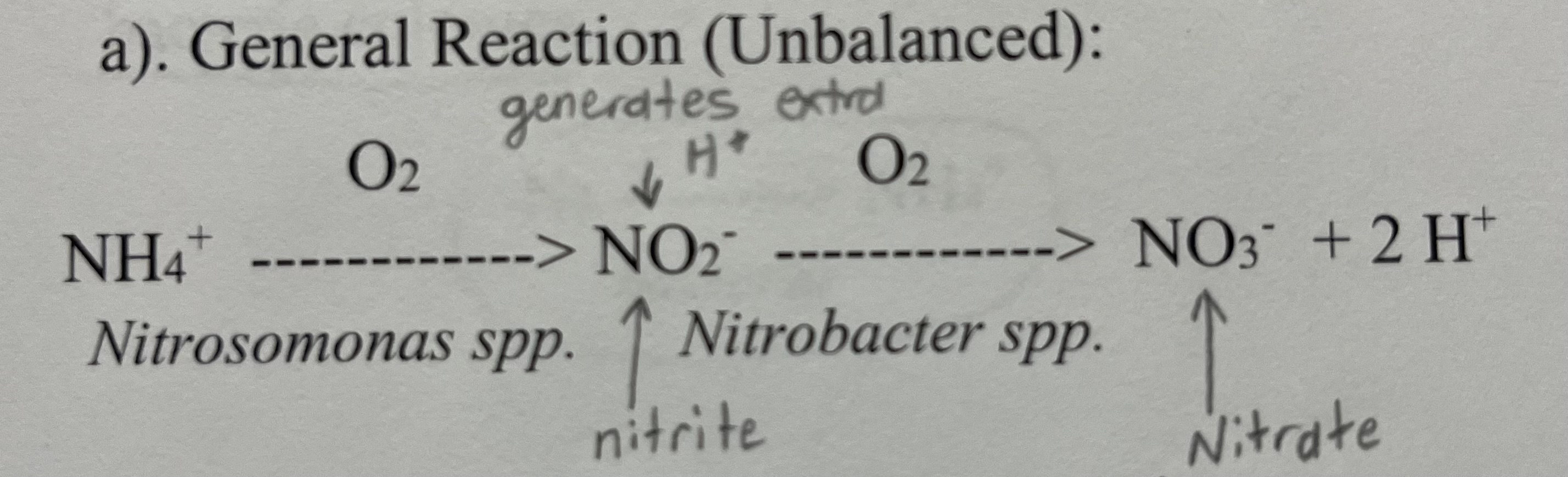
Define Nitrification
the sequence of microbial oxidation processes converting NH4+ to NO3-
Define Volatilization
the loss of NH3 (gas) after amendments containing the N nutrient are applied
Define Denitrification
the loss of available soil Nitrogen by the microbial reduction of NO3- to N2O or N2
Define Leaching
the movement of soil NO3- down through the soil profile with the movement of a wetting front
Define Biological N Fixation
the biochemical reduction of atmospheric N2 to ammonium-N within roots of a host plant by infecting species specific bacteria colonies
Nitrogen Forms in Nature
Gas: NH3 or N2
Organic: R-NH2
Ions (Plant available forms): NO3- or NH4+
Use of Nitrogen in Plants
Proteins, DNA, RNA, Chlorophyll
Most N in plants is converted to Organic amine forms (R-NH2)
What is the importance of the N cycle compared to other nutrient cycles?
N is the plant nutrient in greatest demand
Soil N is involved with the largest number of nutrient cycle processes
Soil N exists in many chemical forms simultaneously
What are the main nutrient cycle components?
Gains (inputs), Losses (outputs), and Transformations (cycling)
Examples of Gains in a nutrient cycle
Biological fixation, Fertilizer/Industrial fixation, Electrical fixation
Examples of Losses in the Nitrogen nutrient cycle
Leaching, Volatilization, Denitrification
Examples of Transformations in a nutrient cycle
Mineralization, Immobilization, Nitrification
What are the reaction products of the nitrification chemical process?
NO3- (Nitrate) and H+
High vs Low C:N and its affects on Mineralization
High C:N - anything >25:1, Lack of Nitrogen limits microbial growth, OM not easily decomposed
Low C:N10:1 to 25:1, Nitrogen is not limiting to microbial growth, OM is easily decomposed.
Trends in Nitrogen release after adding various C:N materials.
High C:N - when incorporated, observe a mid-long term decline in available soil N (think about corn cobs)
Low C:N - when incorporated, observe a short term decline in soil N, then a release of available Nitrogen (think about alfalfa straw)
What is the reaction product of mineralization chemical processes?
NH4+
What are the mechanisms and conditions responsible for denitrification?
Mechanisms: O2 is severely restricted to microbes, anaerobic respiration mechanisms are activated in microbes, NO3-N becomes the electron acceptor instead of O2, bacterial chemically reduce the oxidized N forms present
Conditions:Soil saturation for more than 2 days, soil with poor drainage and high compaction
What are practices to reduce denitrification potential?
Minimize compaction (reduce trips across the field, drive in wheel tracks)
Improve drainage (install tiles or use cover crops)
Monitor irrigation levels
What conditions responsible for enhancement of leaching?
Excess irrigation and high rainfall
Course textured soils
Mismanagement of N fertilizer applications
What practices are used to reduce leaching potential?
Apply majority of Nitrogen in the Spring
Split application throughout the season
Add nitrification or urease inhibitors
Manage irrigation more effectively
Benefits of the symbiotic relationships in biological N fixation.
Plant roots provide carbon and protection to bacteria colonies
Bacteria produce more NH4+ than they require
Excess Nitrogen diffuses into the plant’s xylem stream
What are some management practices to enhance N production effectiveness in biological N fixation?
Inoculate with species specific bacteria
Inoculate seeds at planting time rather than inoculating early and storing
What are the popular N fertilizers.
Urea
Urea-Ammonium Nitrate Solution (UAN)
Ammonium phosphates (DAP and MAP)
Anhydrous Ammonia (cheapest per lb of N, highest concentration of N)
Effective management practices/strategies of N fertilizer materials
Incorporate with light tillage if possible
apply before .25” rainfall (or more) or irrigate
avoid surface applications on grass/pasture or crop residue without irrigation or rainfall
limit application on calcareous soils
What are cautions associated with anhydrous ammonia for biological systems?
NH3 is toxic to biological systems
NH3 reacts with water in mucous membranes, eyes, and lungs. Greater than 0.7% NH3 air concentration = human suffocation and death.
Free NH3 gas can injure seedlings and roots
What are the environmental risks with N fertilizer mismanagement?
Water pollution (algal blooms, dead zones, and groundwater contamination)
Air pollution (ammonia and ozone)
Climate change (greenhouse gas emissions like nitrous oxide)
Ecosystem damage (loss of biodiversity, soil acidification, and degraded habitats)
Mineralization Chemical Reaction
Nitrification Chemical Reaction
Urea Hydrolysis Reaction
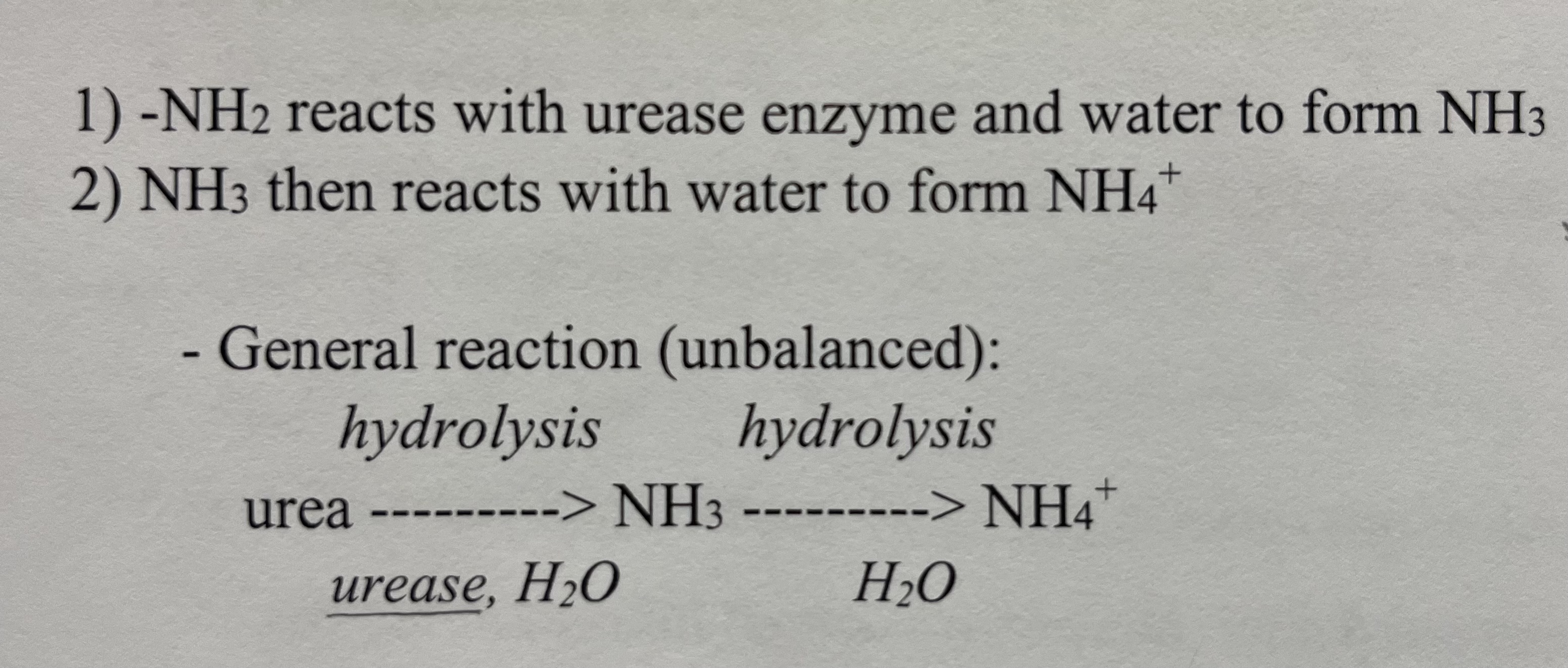
Ammonia Hydrolysis Reaction
