3.1.1 - atomic structure: MS answers, things i learnt from PPQs, etc.
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
31 Terms
how to calculate mass of an ion / isotope in kg
divide 1 by avogadro’s constant
multiply ans. by mass number
this ans will be in g. if kg needed, divide by 1000
Explain how ions are accelerated, detected and have their abundance determined in a time of flight (TOF) mass spectrometer. (3)
(Ions accelerated by) attraction to negatively charged plate / electric field
Ions detected by gaining electrons
Abundance determined by (size) of current flowing
(or amount of electrons gained) in the detector
This question is about Period 3 of the Periodic Table.
(a) Deduce which of Na+ and Mg2+ is the smaller ion. Explain your answer.
Magnesium
Because Mg2+ has more protons
AND
With the same shielding/screening/electron arrangement/number of electrons
Describe how ions are formed in a time of flight (TOF) mass spectrometer (2)
A high voltage is applied to a sample in a polar solvent
the sample molecule, M, gains a proton forming MH+
OR
(for electron impact ionisation)
the sample is bombarded by high energy electrons
the sample molecule loses an electron forming M+
A TOF mass spectrometer can be used to determine the relative molecular mass of molecular substances.
Explain why it is necessary to ionise molecules when measuring their mass in a TOF mass spectrometer. (2)
Ions, not molecules, will interact with and be accelerated by an electric field
Only ions will create a current when hitting the detector
do the chemical properties of isotopes differ or not?
why / why not?
they do not differ
because they have the same e- configuration
Magnesium exists as three isotopes: 24Mg, 25Mg and 26Mg
In terms of sub-atomic particles, state the difference between the three isotopes of magnesium. (1)
They have different numbers of neutrons
Write an equation, including state symbols, to show how an atom of titanium is ionised by electron impact (1)
equation: Ti(g) → Ti+ (g) +e−
Write an equation, including state symbols, to represent the process that occurs when the third ionisation energy of manganese is measured. (1)
Mn2+ (g) ⟶ Mn3+ (g) + e −
State which of the elements magnesium and aluminium has the lower first ionisation energy.
Explain your answer. (3)
M1: Al
M2: (Outer) electron in (3)p sublevel / orbital
Not just level or shell
M3: Higher in energy / further from the nucleus so easier to remove OWTTE
Both required for M3
Ignore shielding
Explain how ions are detected and relative abundance is measured in a TOF mass spectrometer. (2)
M1: ion hits the detector / negative plate and gains an electron
M2: (relative) abundance is proportional to (the size of) the current
State two differences between the ‘plum pudding’ model and the model of atomic structure used today. (2)
(written as if its about the current model)
M1: (Central) nucleus contains protons and neutrons.
M2: Electrons are now arranged in energy levels/shells/orbitals
Ignore “mostly empty space”
Ignore electrons surround / orbit nucleus
Describe the process of electrospray ionisation.
Give an equation to represent the ionisation of P in this process. (4)
DESCRIPTION
M1: P dissolved in to a solvent
M2: (injected through) a needle at high voltage
M3: Gains a proton / H+
EQUATION
M4: P + H+ → PH+
The first ionisation energies of the elements in Period 2 change as the atomic number increases.
Explain the pattern in the first ionisation energies of the elements from lithium to neon. (6)

Time of flight (TOF) mass spectrometry is an important analytical technique.
A mixture of three compounds is analysed using a TOF mass spectrometer.
The mixture is ionised using electrospray ionisation.
The three compounds are known to have the molecular formulas:
C3H5O2N
C3H7O3N
C3H7O2NS
Describe how the molecules are ionised using electrospray ionisation . (3)
M1: (Sample is) dissolved (in a volatile solvent)
M2: (Injected through) needle at high voltage
M3: Each molecule gains a proton/H+
(for the same question as the last flashcard) Give the formula of the ion that reaches the detector first in the TOF mass spectrometer. (1)
C3H6O2N+ / C3H5O2NH+
Must be charged
Explain why the atomic radius decreases across Period 3, from sodium to chlorine (2)
M1: nuclear charge increases
M2: shielding is similar/same
Identify the element in Period 3, from sodium to chlorine, that has the highest electronegativity (1)
Chlorine
Give the meaning of the term relative atomic mass (2)
M1: the average mass of one atom of an element
M2: compared to 1/12 of the mass of an atom of 12C (carbon-12)
The sample of chromium is analysed in a time of flight (TOF) mass spectrometer.
Give two reasons why it is necessary to ionise the isotopes of chromium before they can be analysed in a TOF mass spectrometer. (2)
M1: (Ions will interact with and) be accelerated (by an electric field)
M2: Ions create a current when hitting the detector
There is a general trend for an increase in ionisation energy across Period 3.
Give one example of an element that deviates from this trend.
Explain why this deviation occurs. (3)
M1: Element = Aluminium
M2: (Outer) electron in (3)p orbital / sub-shell (level)
M3: (3p) higher in energy / slightly more shielded (than 3s) / slightly further away (than 3s)
OR YOU CAN TALK ABOUT SULFUR
M1: Sulfur
M2: (Outer) electrons in (3)p orbital begin to pair
M3: Repel
define the mass no. of an atom (1)
Number of protons + neutrons (in the nucleus of the atom)
State how the relative abundance of 185Re+ is determined in a TOF mass spectrometer (2)
M1: at the detector/(negative) plate the ions/Re+ gain an electron
M2: (relative) abundance depends on the size of the current (i.e. current is proportional to abundance)
Electrospray ionisation is used instead of electron impact for the ionisation of a protein in a mass spectrometry experiment.
Suggest why. (1)
the protein (ion) does not break up/fragment
A mixture of gases is analysed using TOF mass spectrometry. The mixture contains argon, carbon dioxide, nitrogen and oxygen. The mixture is ionised by electron impact.
State the meaning of the term electron impact ionisation. (1)
(High energy) electrons (from an electron gun) are used to knock out an electron (from each molecule or atom.)
(generalised question) why would a certain ion reach the detector last (1)
Has the highest mass (to charge ratio) (so will travel the slowest)

question in pic

State how the detector enables the relative abundance of each ion to be determined. (1)
The (relative) abundance is proportional to the size of the current
This question is about the elements in Group 2.
Explain why the third ionisation energy of beryllium is much higher than the second ionisation energy of beryllium. (3)

(dk if this is actually for this topic, but carries on from last question) Magnesium reacts slowly with cold water but rapidly with steam.
Compare these reactions, in terms of the products formed. You should identify one similarity in, and one difference between, these reactions (2)
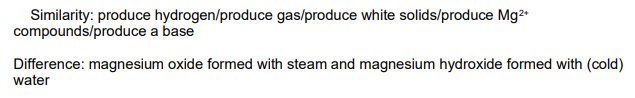
The reaction of calcium with water is a redox reaction.
Explain, in terms of oxidation states, why this reaction involves both oxidation and reduction. (2)
