Mixtures and Solutions
1/26
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
27 Terms
mixture
a combination of 2 or more materials
solute
a substance that dissolves in a solvent to form a solution
solvent
a substance that dissolves a solute to form a solution
solubility
the property that substances have of dissolving in solvents
dissolve
to break down into smaller pieces
evaporation
causes liquids to dry up--the liquid turns to gas and disperses into the air, leaving any dissolved solid material behind
insoluble
Describes a substance that cannot be dissolved in a given solvent,
Not able to be dissolved.
Solution
a special mixture where one substance dissolves into another
filter
a tool used to separate solids and liquids and different size solids from one another.
magnet
tool used to separate iron from a mixture
Colloid
A mixture containing small, undissolved particles that do not settle out.
Suspension
A mixture in which particles can be seen and easily separated by settling or filtration
a rise in temperature
makes solids more soluble but not gasses
a rise in pressure
makes gasses more soluble
concentration
A measurement of how much solute exists within a certain volume of solvent
What is filtration?
The process in which solid particles are removed from a liquid/gas by use of a filter
What is distillation?
A separation process that involves heating a liquid in a mixture of liquids so it evaporates and then condenses, separating the liquids.
What is chromotography?
A separation technique in which a mixture is mixed with a gas or liquid (such as water) in which the components in that mixture travel at different speeds through/react with differently. This leads to the components in the mixture to separate
What is chromatopgraphy typically used to separate?
Coloured dyes that have been mixed together
What is evaporation used to separate?
A mixture in which part of it was solid (e.g., water and sand)
What is distillation used to separate?
A mixture made purely of liquids
What are immiscible liquids?
Liquids that do not mix at all with each other (e.g., oil and water)
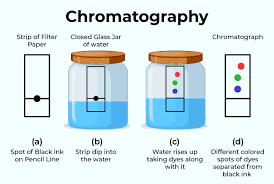
What separation technique does the following diagram show?
Chromatography
What are the 4 main separation techniques?
Filtration, distillation, evaporation and chromatography
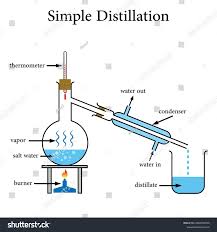
What separation technique does the following diagram show?
Distillation
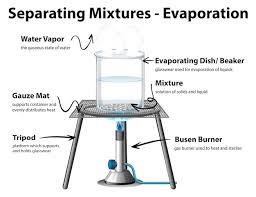
What separation technique does the following diagram show?
Evaporation
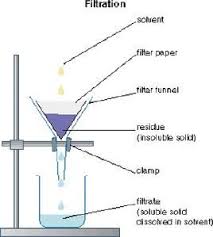
What separation technique does the following diagram show?
Filtration