Reagents Alkenes and Alkynes
1/21
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
22 Terms
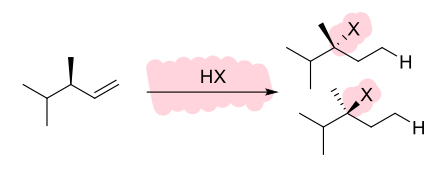
HBr (alkene)
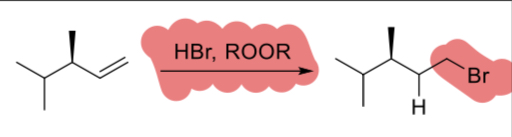
1.HBr
2.ROOR
(alkene)
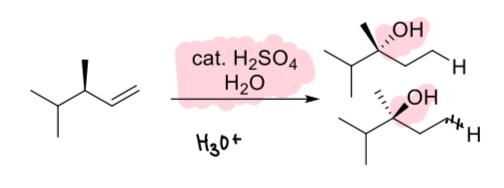
1. H2SO4
2. H2O
(alkene)
Br2/CCl4
(alkene)
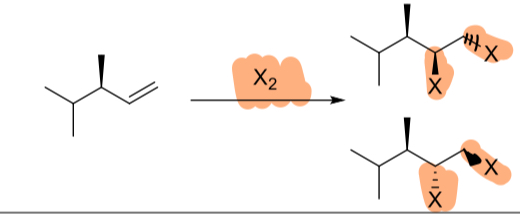
Br2/H2O
(alkene)
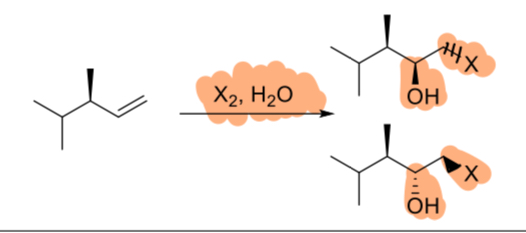
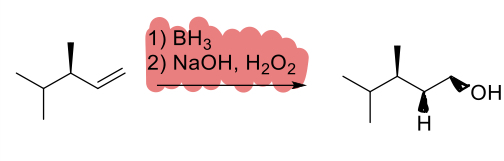
1. BH3
2. NaOH, H2O2
(alkene)
1) mCPBA
2) cat. H2SO4, H2O
(alkene)
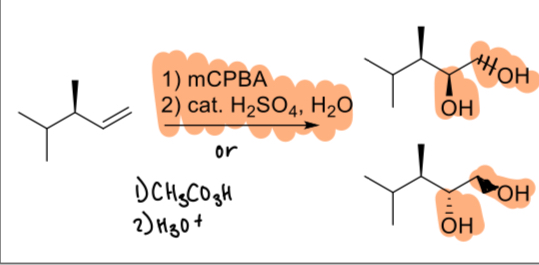
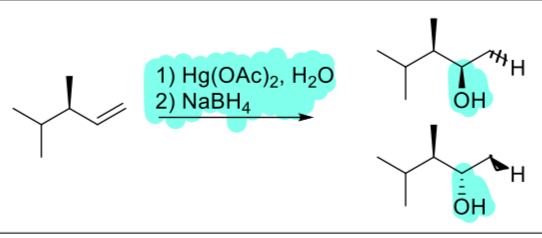
1. Hg(OAc)2, H2O
2. NaBH4
(alkene)
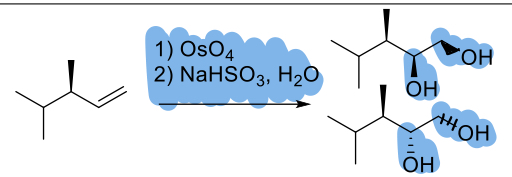
1) OsO4
2) NaHSO3, H2O
(alkene)
1) O3
2) S(CH3)2 aka DMS
(alkene)

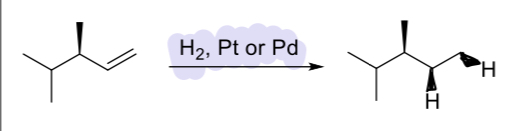
H2, Pt
(alkene)
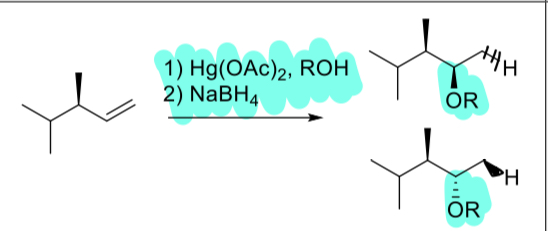
1. Hg(OAc)2, ROH
2. NaBH4
(alkene)
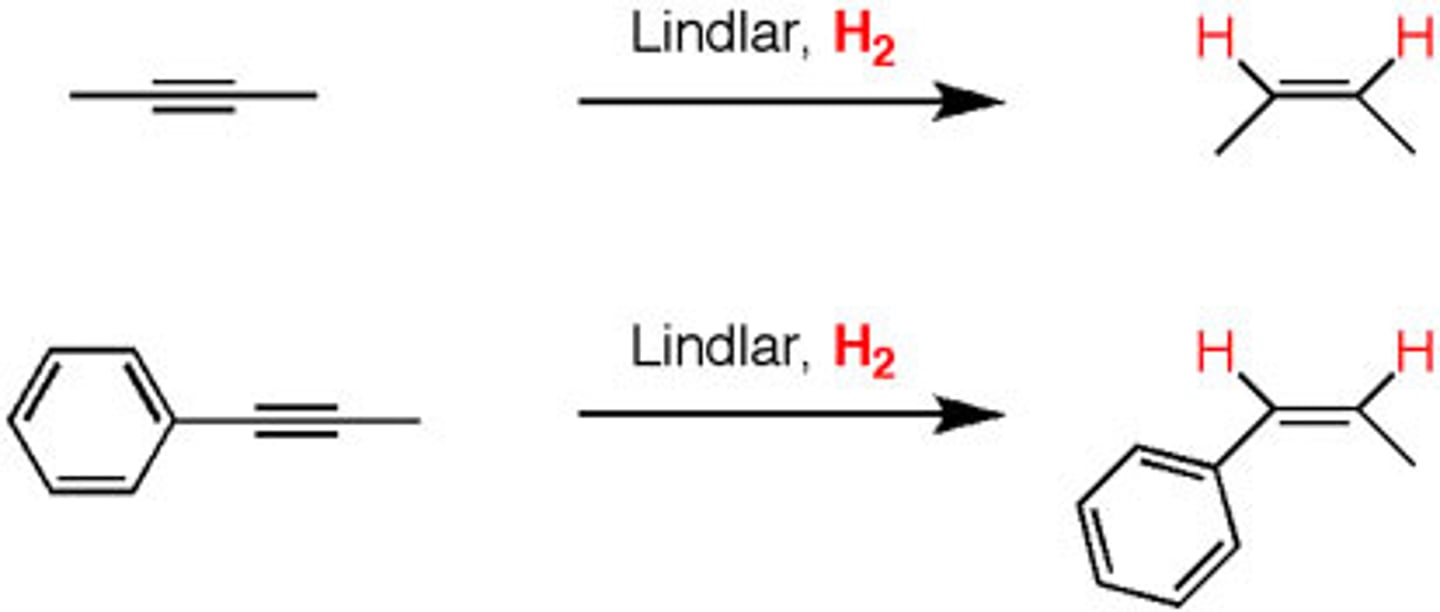
H2, Lindlar's catalyst
(Alkyne)
Z Alkene
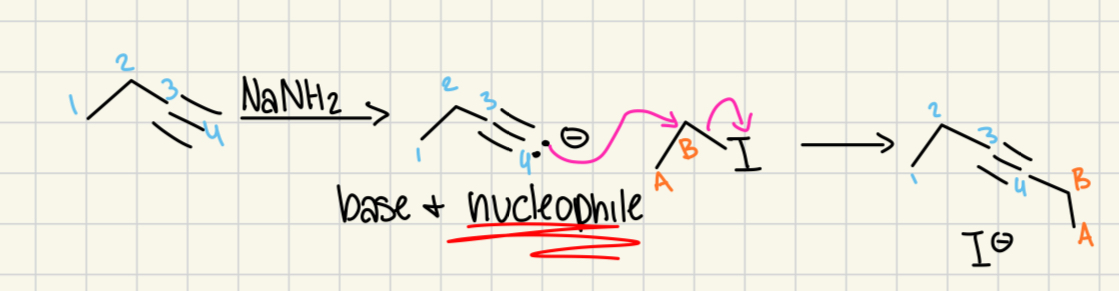
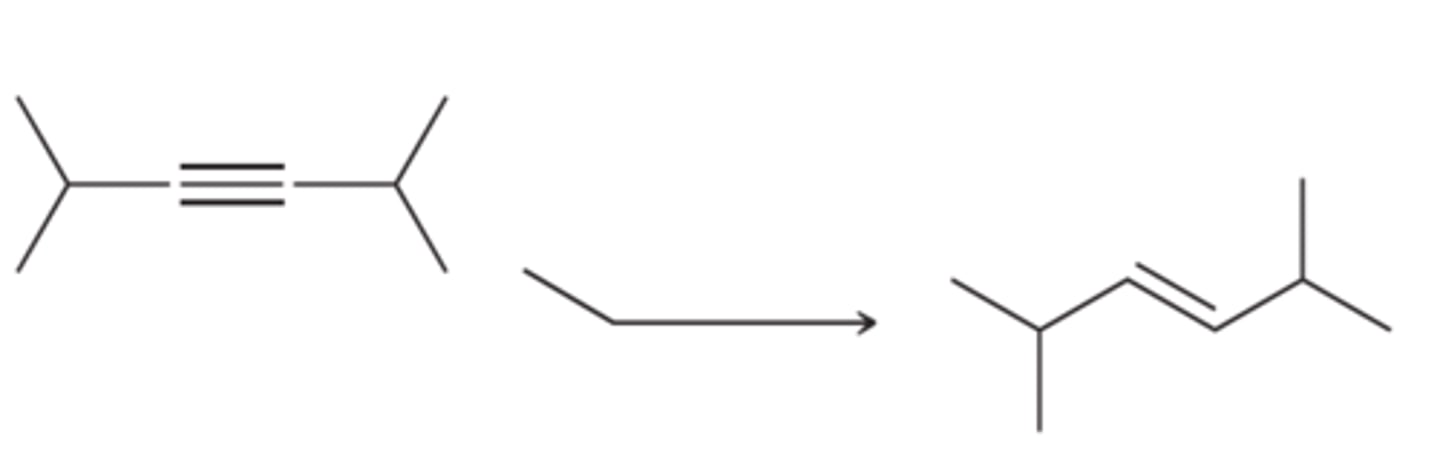
Na/NH3(l)
(Alkyne)
Alkyne to trans alkene.
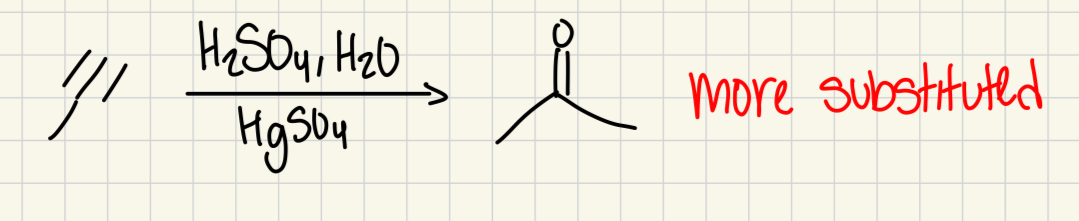
HgSO4, H2SO4, H2O
(Alkyne)
carbonyl on more substituted carbon
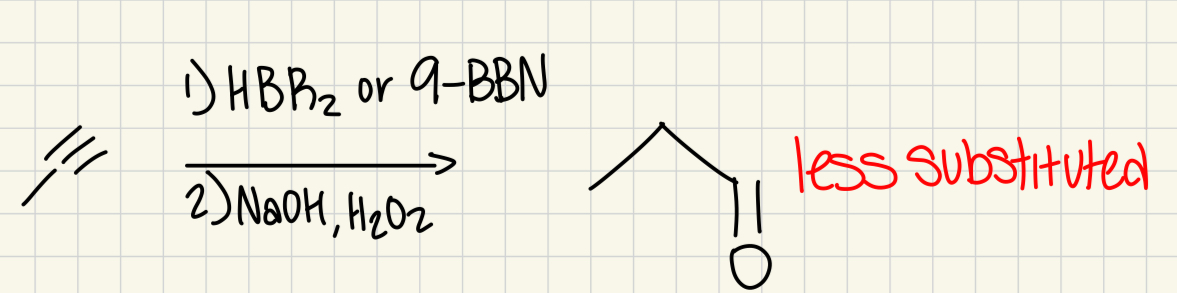
1) HBR2 or 9-BBN
2) H2O2, NaOH
(Alkyne)
carbonyl on less substituted carbon
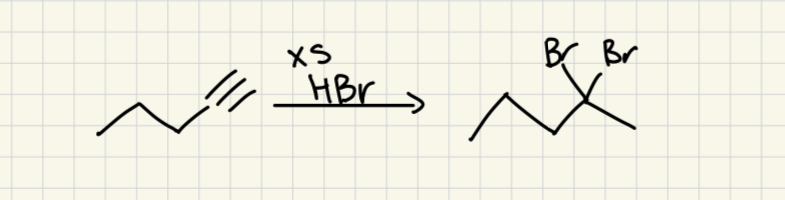
xs HBr
(Alkyne)
2 Br on more substituted carbon
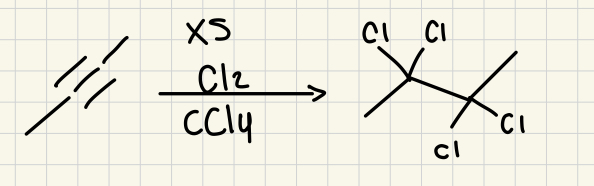
xs Br2, CCl4
(Alkyne)
4 Br added to both ends of alkyne
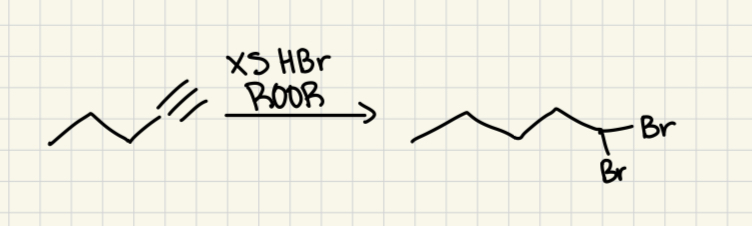
HBr, ROOR
(alkyne)
2 Br on less substituted carbon
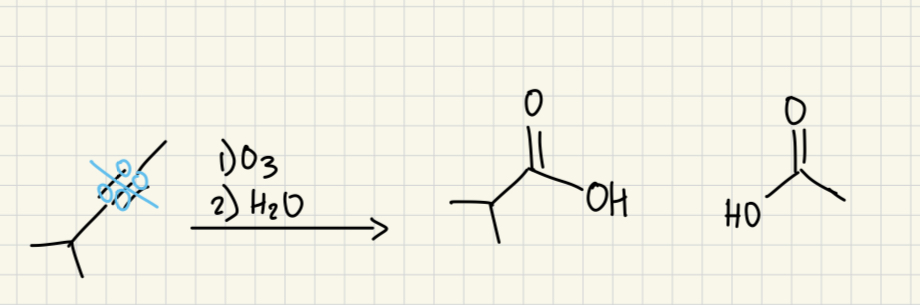
1) O3
2) H2O
(alkyne)
split too carboxylic acid and CO2
1) xs NaNH2 or xs LDA
2) H2O
creates terminal alkyne
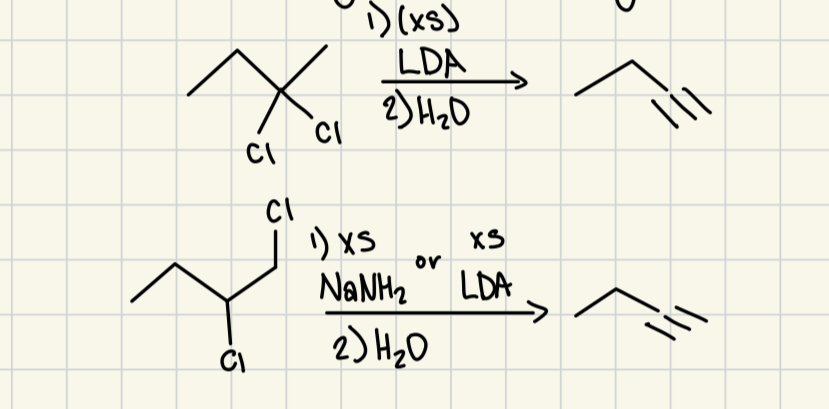
NaNH2
depronating alkyne