4. Lipids and Carbohydrates
1/131
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
132 Terms
What are the functions of lipids?
energy storage (abundant in cells)
structure (abundant in cells)
signalling (lower conc in cells)
How do lipids play a role in animal cells?
plasma membrane provides semipermeable barrier with outside wall
membrane bound organelles creating compartments with different environments
lipid bilayer
What are some characteristics of lipids?
generally non-polar (entirelly or in part)
therefore low solubility in water
How is energy stored in lipids?
fatty acids
Triacylglycerol/triglyceride
waxes
phospholipids
Examples of storage lipids?
fatty acids
triglycerides
waxes
Examples of membrane lipids?
phospholipids
glycolipids
cholesterol
Examples of signalling lipids?
phospholipid derivatives
steroid hormones (cholesterol derivatives)
eicosanoids (paracrine hormones)
lipid soluble vitamins (vitamin A)
What are fatty acids?
carboxylic acids (-1) with hydrocarbon chains containing 4-26 carbons
almost all have an even number of carbon
most natural fas are unbranched
amphipathic (carboxyl = hydrophilic, hydrocarbon chain - hydrophobic)
Difference between saturated, monounsaturated and polyunsaturated
saturated: no double bonds b/w carbons in chain
monounsaturated: one double bond b/w carbons in alkyl chain
polyunsaturated: more than one double bond in alkyl chain
Nomenclature
1) C1 = carboxyl group carbon
2) 18:0 means 18 C and no C=C
3) 18:1 Δ9,12 means 18 C and 2 C=C starting at carbon 9 and carbon 12
saturated fatty acid trends
increasing melting point as increased chain size
increasing melting point as decreasing double bonds
decreasing water solubility as chain size increases
why do fatty acids pack into stable aggregates?
fully saturated C backbone is usually usually in a fully extended conformation
therefore pack into a nearly crystalline array, stabilized by
extensive hydrophobic interactions of the hydrocarbon chain
Why increased size of fatty acid = increased boiling point?
Longer carbon chains require more energy to disrupt the packing (more entropy required to expel water) => higher melting temperatures
cis or trans unsaturated?
double bond usually cis (not trans)
unsaturated fatty acid trends and why?
lower melting point
Unsaturated cis fatty acids pack less orderly due to the kink → less-extensive favourable interactions
It takes less thermal energy to disrupt disordered packing of unsaturated fatty acids
How do trans fatty acids form?
partial dehydrogenation of unsaturated fatty acids
done to increase shelf life or stability at high temperature of oils used in cooking
trans double bonds allows…
adopt an extended conformation
trans vs. cis fatty acids
pack more regularly and show
higher melting points than cis forms
trans or cis fatty acids good for you?
trans increases risk of cardiovascular disease
Why is olive oil a liquid while butter is a soft solid?
composition of saturated and unsaturated fatty acids
olive oil = more C18 (unsaturated) therefore disrupted packing and more space between molecules - liquid
butter = lower C16/C18 unsaturated and more C16/C18 saturated fatty acids, pack more easily therefore a solid
What are omega-3 fatty acids?
essential nutrients, humans can’t synthesise
ALA, DHA, EPA
can fatty acid double bonds be conjugated?
no! must be separated by C=C-C-C=C
Where does the Omega-3 name come from?
omega = last letter of greek alphabet
therefore C1 is the last C (opposite end to COOH)
therefore C3 is the first double bond
What are triacylglycerols?
fat molecule, glycerol backbone and three acyl trains
glycerol OH’s provide 3 sites for ester linkages (carboxylic acid reacts with hydroxyl)
HYDROPHOBIC
provide stored energy and insulation
What is glycerol?
three carbon alcohol
simple vs. complex triacylglycerols
simple: all three fatty acids identical
complex: fatty acids differ
What are advantages of triacylglycerols?
higher energy yield than oxidation of other fuel sources such as glycogen or starch
not hydrated (less weight)
What are adipocytes?
fat cells
lipid bilayer
two sheets
hydrophobic tails in middle
hydrophilic heads outside
proteins and sugars
channels and pores
glycoproteins
sterol
sphingolipids
phospholipids
phospholipids
phosphate group in their polar head
two non-polar tails
two types:
glycerophospholipids (glycerol)
sphingolipids (sphingosine)
difference b/w glycerophospholipids and glycerophospholipids?
one polar head group + 2 non-polar tails
g: uses glycerol
s: uses sphingosine
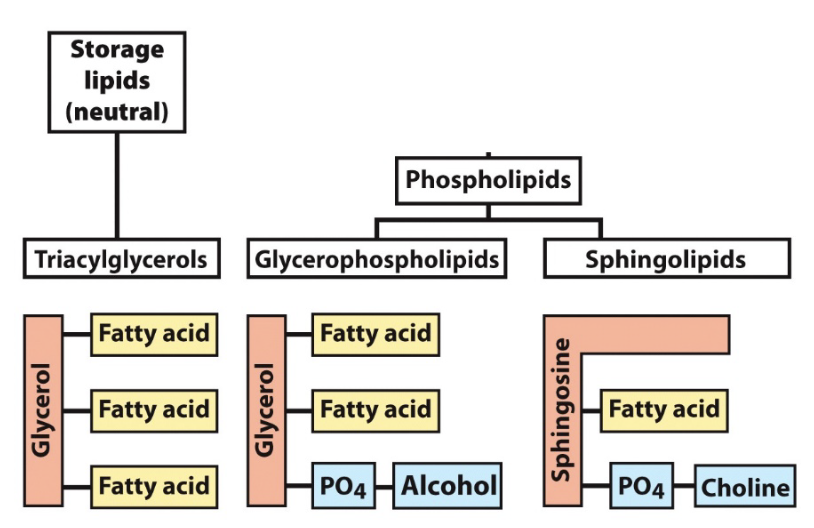
most common glycerophospholipid?
phosphatidylcholine
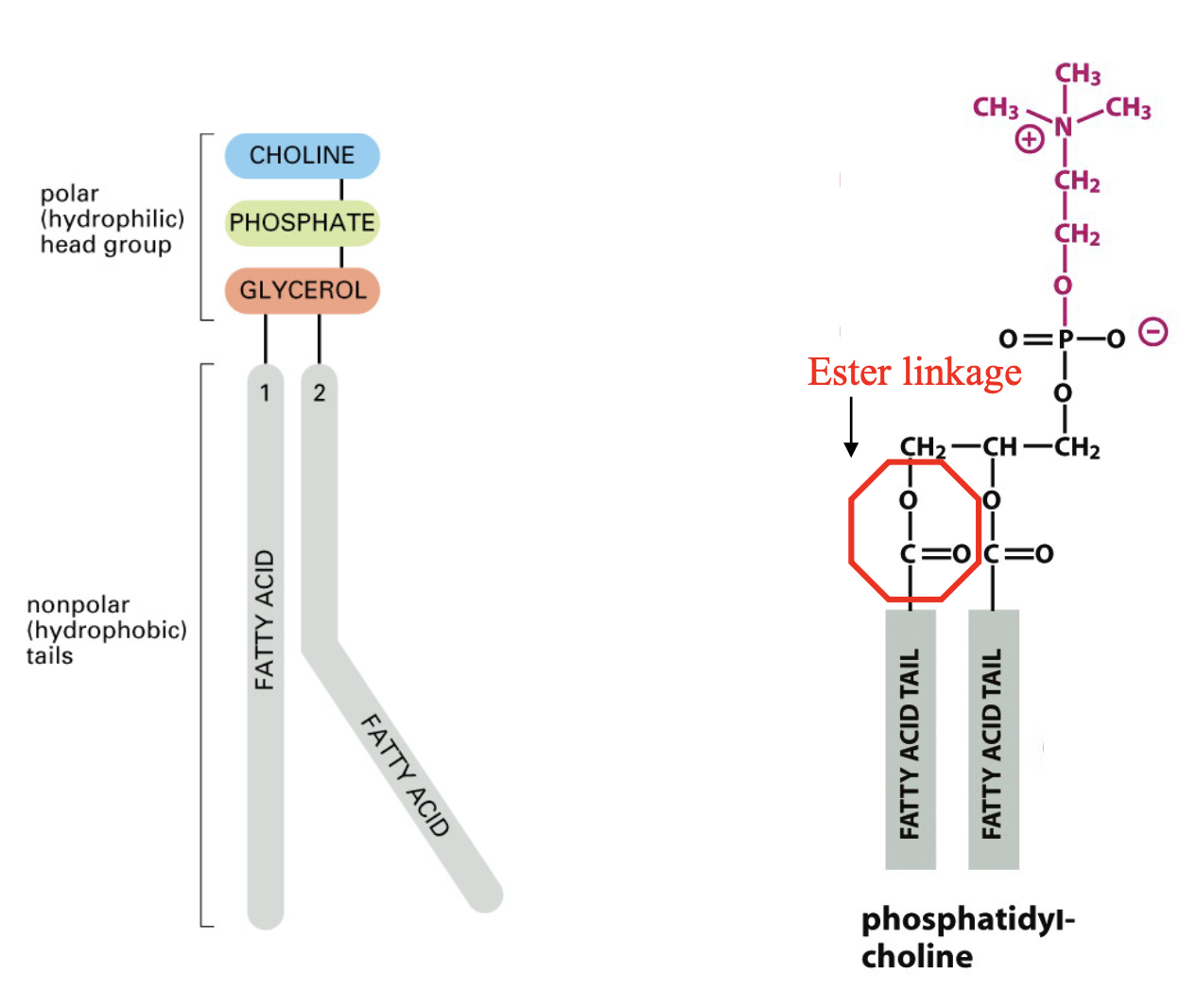
glycerophospholipids
further defined by additional components in head group
ie. -H is phosphatic acid, -NH3 is …
charges on head range from -4 - 0
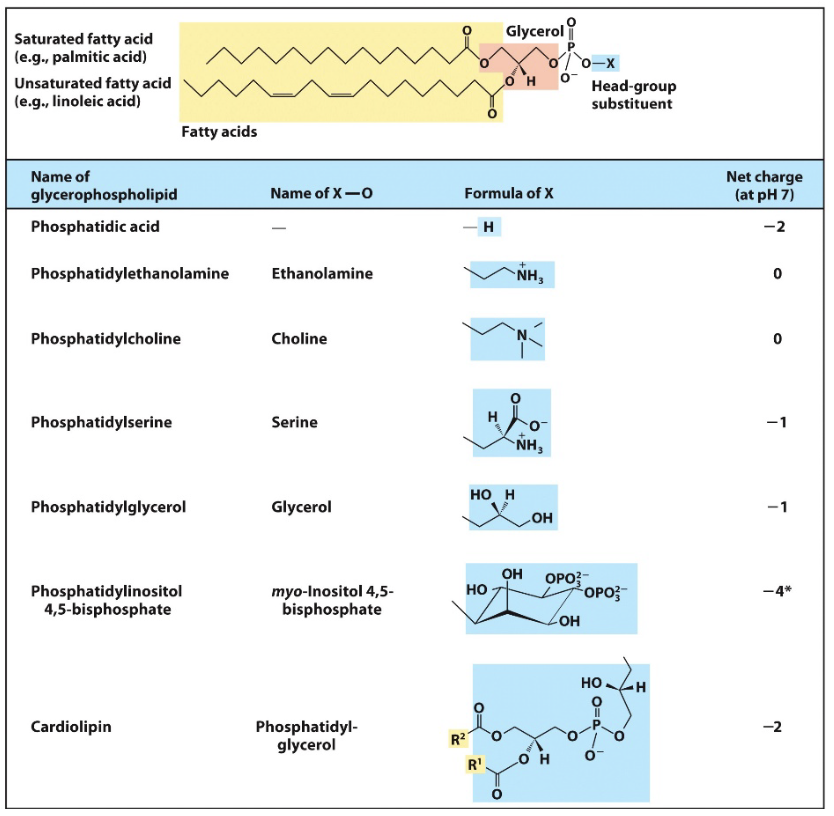
sphingolipids
backbone derives from amino alcohol sphingosine
carbons 1,2,3 of backbone considered equivalent to the 3 carbon glycerol but also contributes 1 of two tails
simplest is ceramide, X of alcohol is H
DONT ALWAYS CONTAIN A PHOSPHATE GROUP
glycolipids
structural and signalling
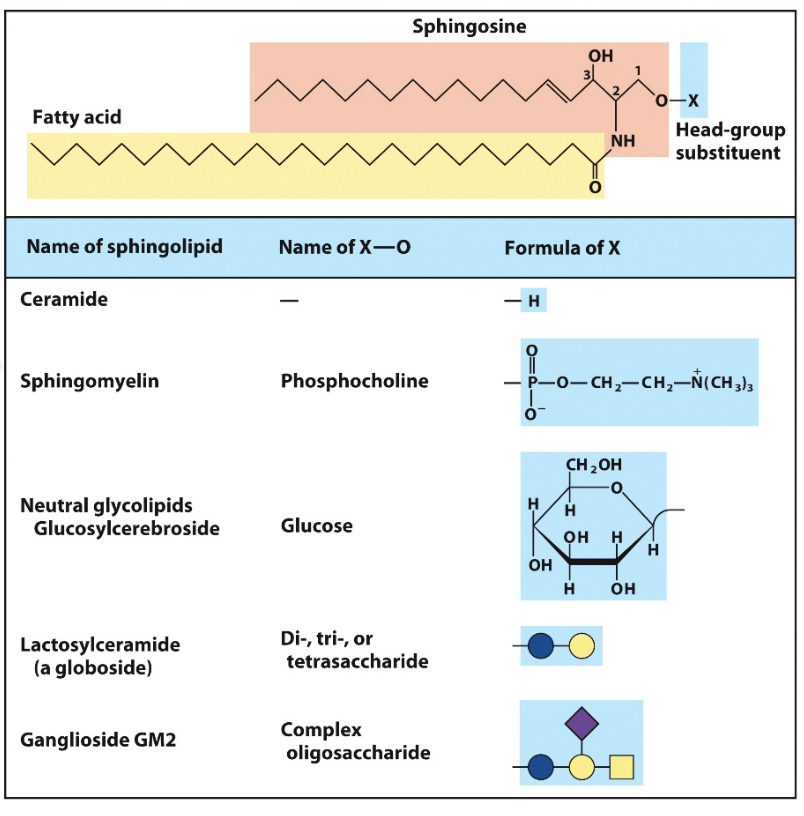
glycolipids
mono or oligosaccharaide unites in head groups
lipid with sugar
sphingolipids
galactolipids (sulfolipids)
common on OUTER membrane
sterols
four fused rings decorated with alkyl side chains
cholesterol
polar head group (OH) on carbon ring
structural, signalling precursor
biological membranes are…
lipid bilayers
thickness of membranes?
3nm
what is the fluid mosaic model of membranes
incorporates proteins within the lipid bilayer
proteins embedded within the bilayer are held by hydrophobic interactions
interactions among components are non-covalent (allowing fluid, dynamic properties)
charges of the lipid head groups contribute significantly to surface properties of membranes
what shape do fatty acids form?
micelles (conical individuals)
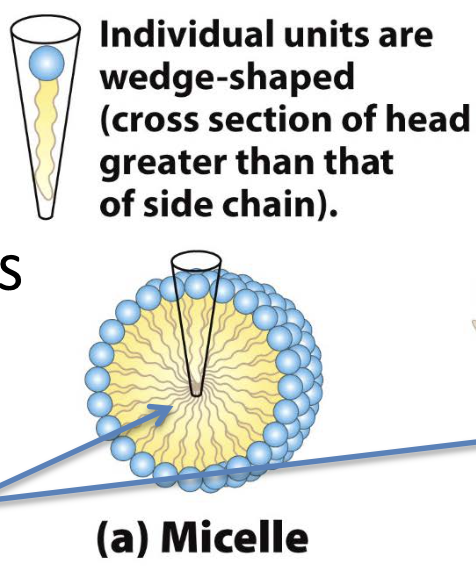
what shape do phospholipids form?
bilayer (cylindrical individuals)
exposed hydrophobic region on edges are exposed to water → unstable
bilayers fold to dorm hollow vesicle (liposome)
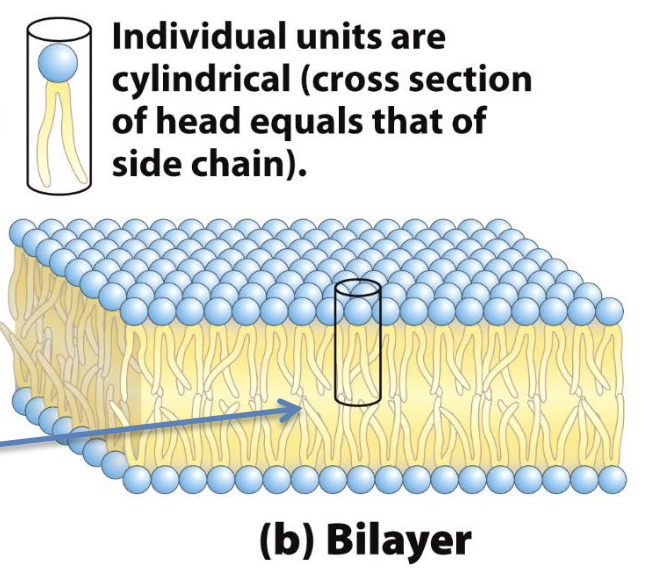
what is the aqueous cavity?
what impacts the membrane fluidity?
fatty acid composition
length of fatty acyl chains
degree of unsaturation
more saturated = better packing = more rigid
cholesterol content
moderates membrane fluidity
high conc = stiffens
low conc = breaks up packing = more fluid
membrane and temp
37ºC = all biological membranes are fluid
phase transition temperature - temp at which membrane goes from paracrystaline state to fluid state
increased temp = more fluid
lipid movement
lateral diffusion very fast at 37ºC
transverse diffusion (flip-flop) is very slow
therefore monolayers can have different lipid compositions
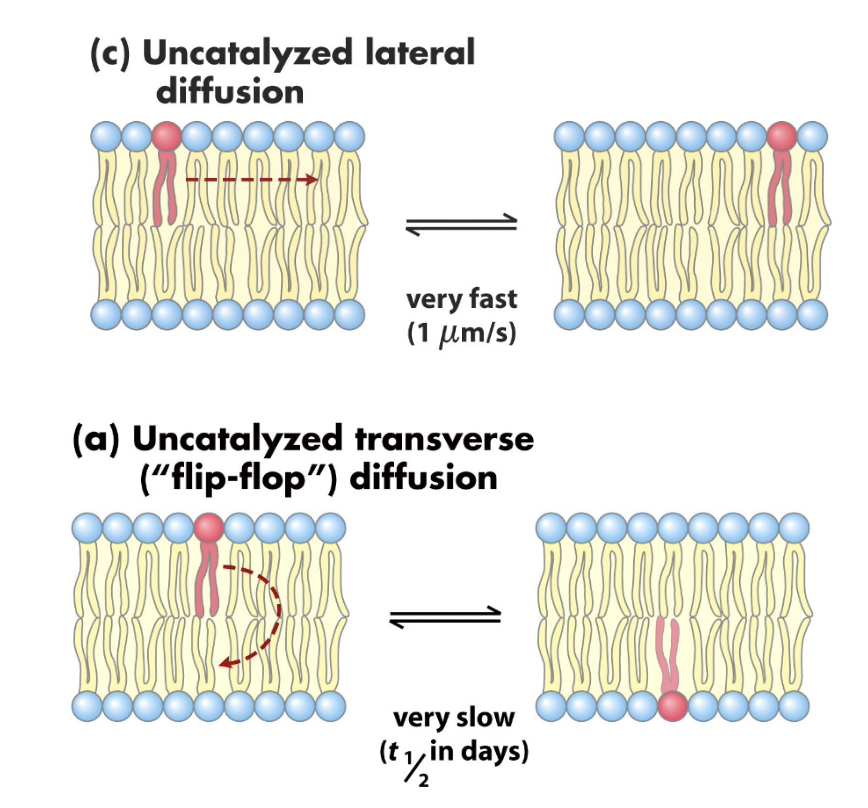
lateral diffusion
measured experimentally by fluorescence recovery after photobleaching (FRAP)
membrane rafts
sterols and sphingolipids cluster together in membrane rafts/lipid rafts/micro domains
slightly thicker, more ordered (less fluid) and harder to dissolve in non-ionic detergents than surrounding region)
behave like a liquid-ordered RAFT in the SEA of liquid-
disordered phospholipids
function of membrane proteins
transporters (selective entry)
receptors for recognition signals
provide structural support
can proteins move around the membrane like lipids?
yes! they are free to diffuse laterally
how do proteins interact with membrane?
integral membrane protein
peripheral protein
integral membrane proteins
deeply embedded in the membrane - firmly attached
strong hydrophobic interactions b/w amino acids on surface of protein and acyl chain of lipids
peripheral membrane proteins
associate with outside surfaces, not attached/embedded
ionic interactions and H-bonding with:
polar head group of lipids
integral membrane proteins
how can peripheral membrane proteins be released?
interact non-covalently therefore released by reagents that disrupt ionic interactions
high salt (salt bridges)
change pH
chelating agent
how can integral membrane proteins be released?
reagents that disrupt hydrophobic interactions eg. detergents like SDS (sodium dodecyl sulphate)
remember extracting DNA from strawberries prac
true or false: all membrane proteins have a unique orientation in the membrane?
true!!
how can you determine orientation/arrangement of membrane proteins? ie which bits are inside/outside
protease sensitivity of proteins from intact cells
enzyme which chops proteins up, to determine which bits are outside
trans membrane and inside protein are intact
what can we assume about transmembrane proteins?
sequence will consist of hydrophobic amino acids
conformation will be an alpha-helix
span (segment of protein) equal to width of membrane
how many amino acids fit in a membrane?
~ 20 hydrophobic residues
membrane = 3nm thick
alpha-helix = 3.6 residues/turn
each turn 0.54nm length
0.15nm/residue
3/0.15 = 20
what is hydropathy index?
a measure of polarity of each amino acid
+ve value = hydrophobic
-ve value = basic/hydrophilic
hydropothy plot
y axis: hydropathy index
x axis: residue number
+ve values will hydrophobic and be the inside of the membrane
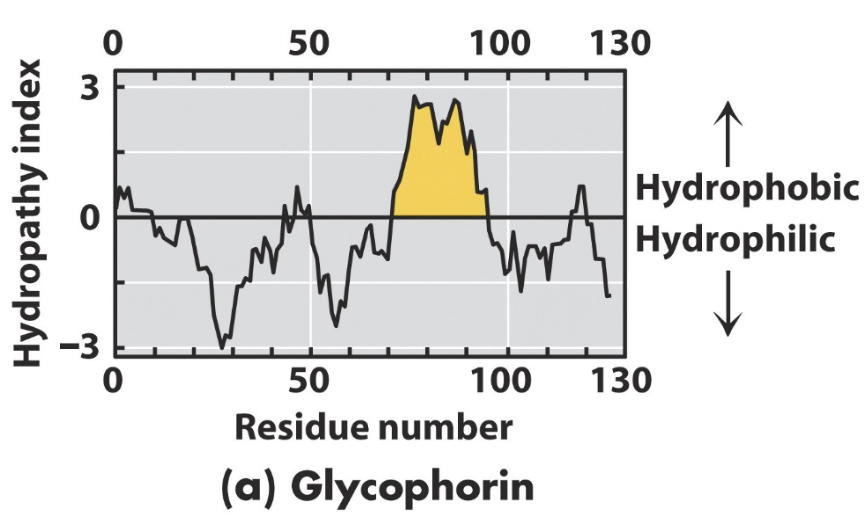
true or false: hydropathy plots can have multiple hydrophobic peaks?
true!
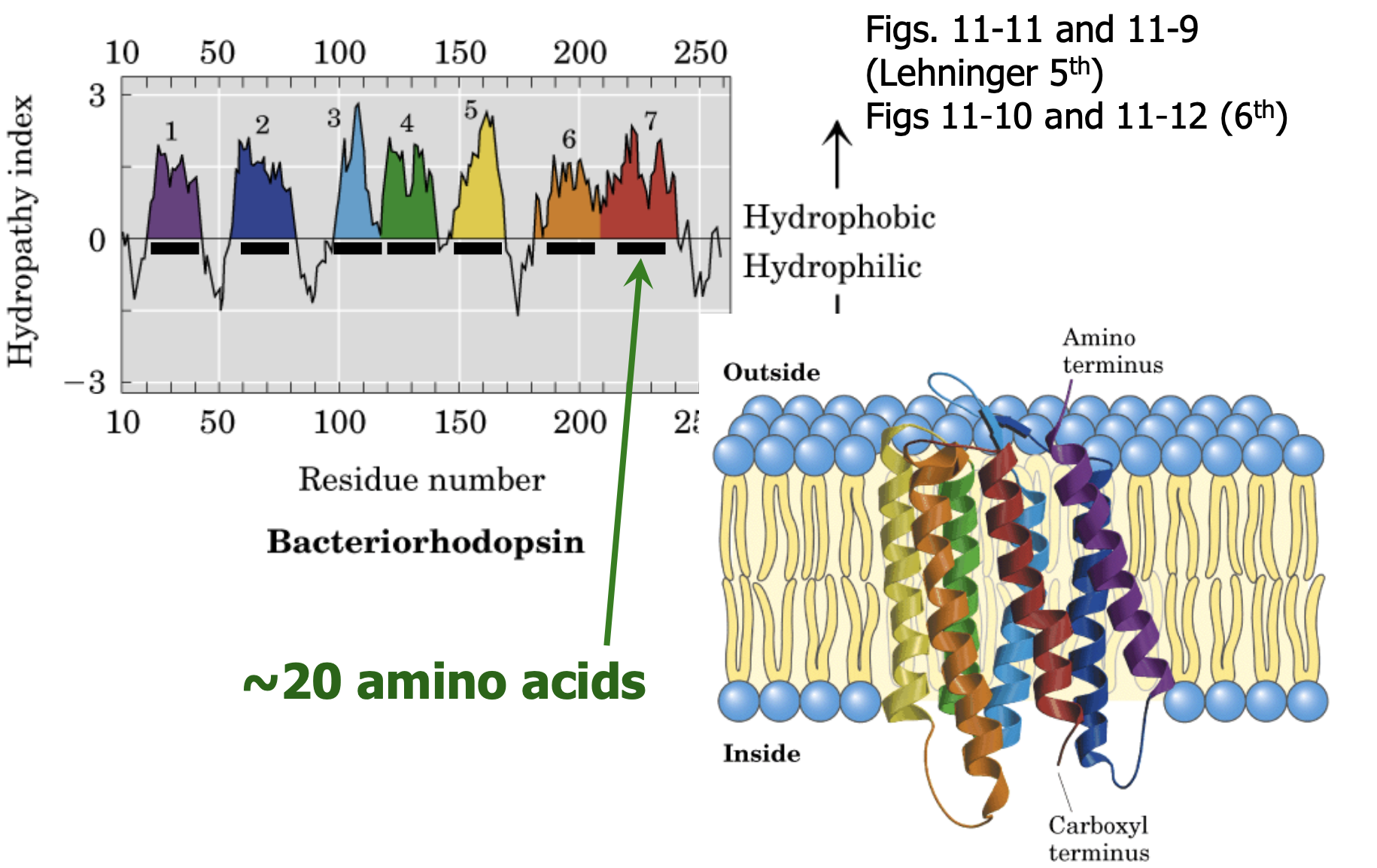
where are the N- and C- termini found?
N/amino = outside
C/carboxyl = inside
how is water and other small molecules transported across membrane?
transport proteins like channel proteins
true or false: transport proteins allow many types of molecules to pass through?
false-ish
each transport protein transfers a particular type of molecule - specific
How are membrane transport proteins classed?
carriers (transporters)
passive/active
channels
passive
high conc to low conc
key feature of carriers
conformational change
open on one side closed on the other → then switch
glucose transporter (GLUT1)
nearly all mammalian cells
passive transport ie. facilitated diffusion down conc gradient
no other substance transported (no fructose/other sugar)
mechanism of glucose transporter
two conformational states (T1 and T2)
T1 open on outside → binding of glucose → conformational change → T2 allows glucose to relase insie cell
also happens other way ie. glucose inside → outside
active transporters
require energy input
transport against conc gradient
eg. ATP powered pumps
P-type ATPases
P-type ATPases examples
sodium ion pump
hydrogen potassium
calcium ATPase
function of carbs/sugars?
source of energy/stored fuels
structure to cells and organisms (cellulose in plants, chitin in arthopods)
cell biology
major component of cell surface
important in influencing function of proteins
important in specific recognition interactions
cell-cell adhesion
how are glycoproteins synthesised?
protein = genes
sugars = ?
monosaccharides
basic unit of carbs
aldehyde or ketones that have two or more hydroxyl groups
[C-H2O]n ‘carbon hyrdate’
three or more carbons
structural formula of monosaccharide
[C-H2O]n
ketone vs. aldehyde?
ketone C=O
aldehyde C(=O)(-H)
ketose vs aldose?
ketose: ketone sugar
aldose: sugar with aldehyde group
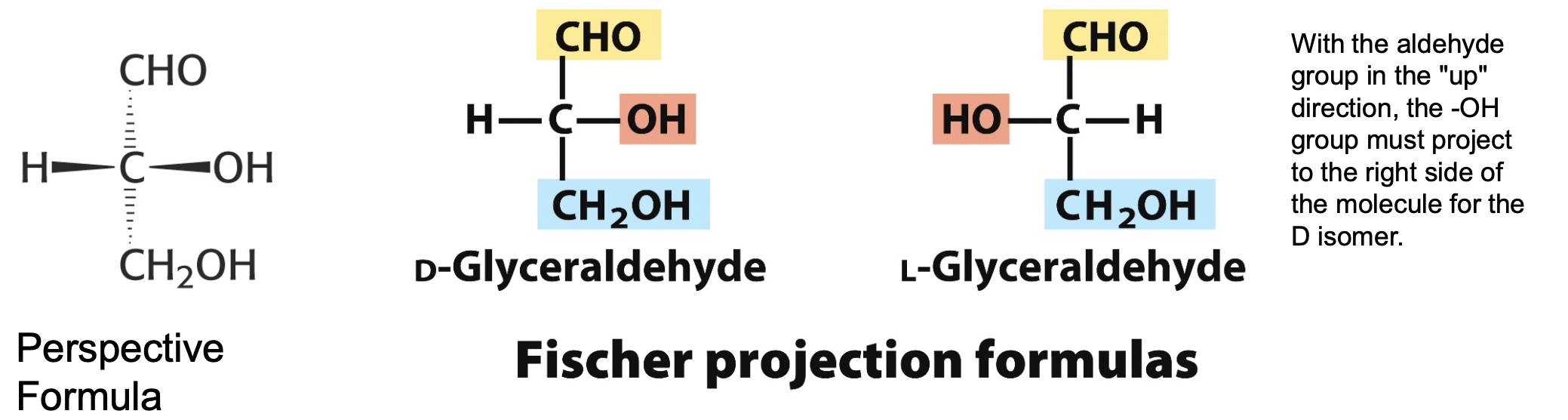
most naturally occurring sugars are what chirality?
D (not L)
chirality as each substituent on C is dfferent
fisher projections
provide a clear and simple view of stereochemistry at each carbon centre
horizontal lines project out of the plan on paper
vertical lines project behind the plane of the paper
monosaccharide nomenclature
three - triose
four - tetrose
five - pentose
six - hexose
seven - heptose
most common monosaccharide in nature
hexose
aldohexoses
four asymmetric chiral centres
D-Aldose monosaccharides
“D” designates the configuration of the asymmetric carbon furthest from the aldehyde group
D-glucose, D-mannose and D-galactose are abundant six carbon sugars
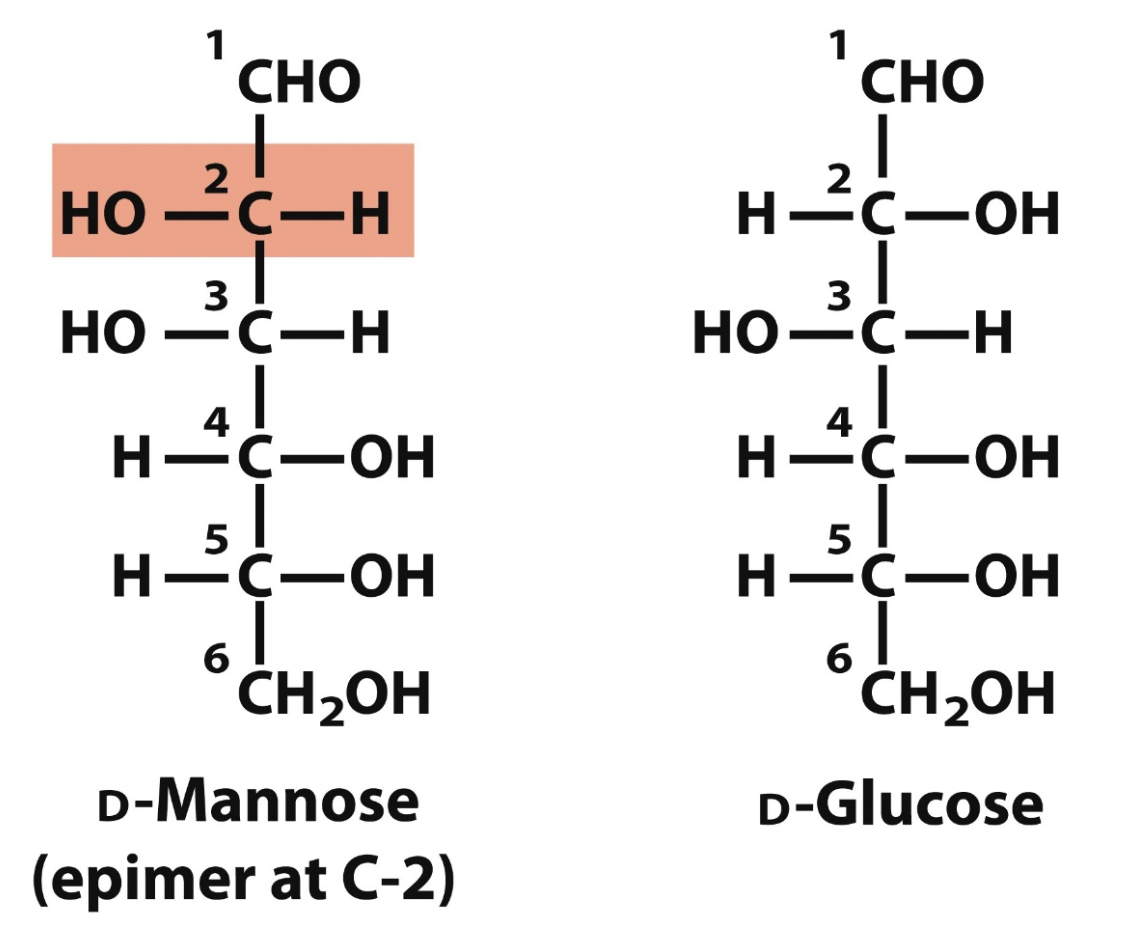
D-glucose and D-mannose differ in configuration only at C-2
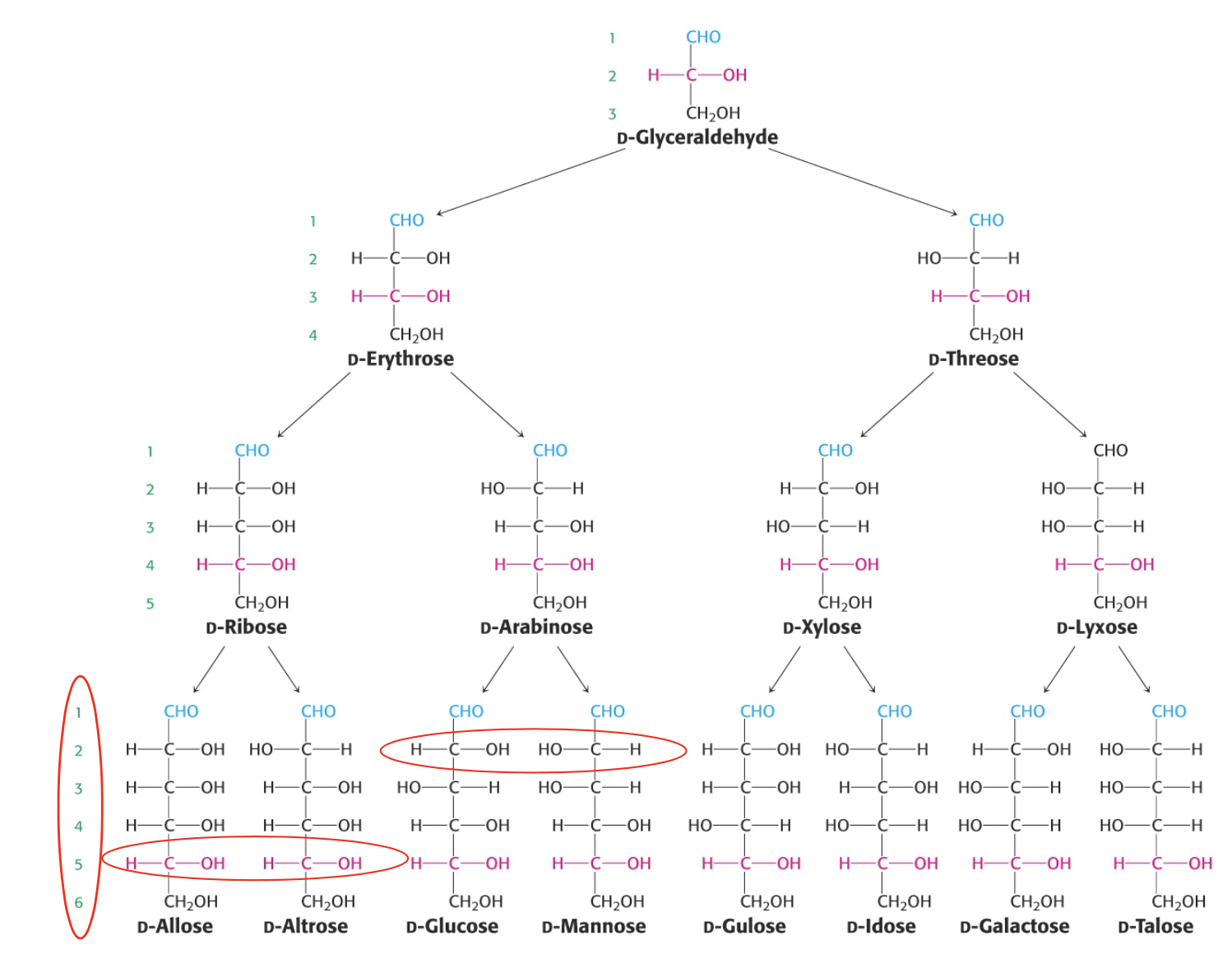
epimers
sugars that differ only in the config around one carbon atom
like D-Mannose and D-Glucose at C2
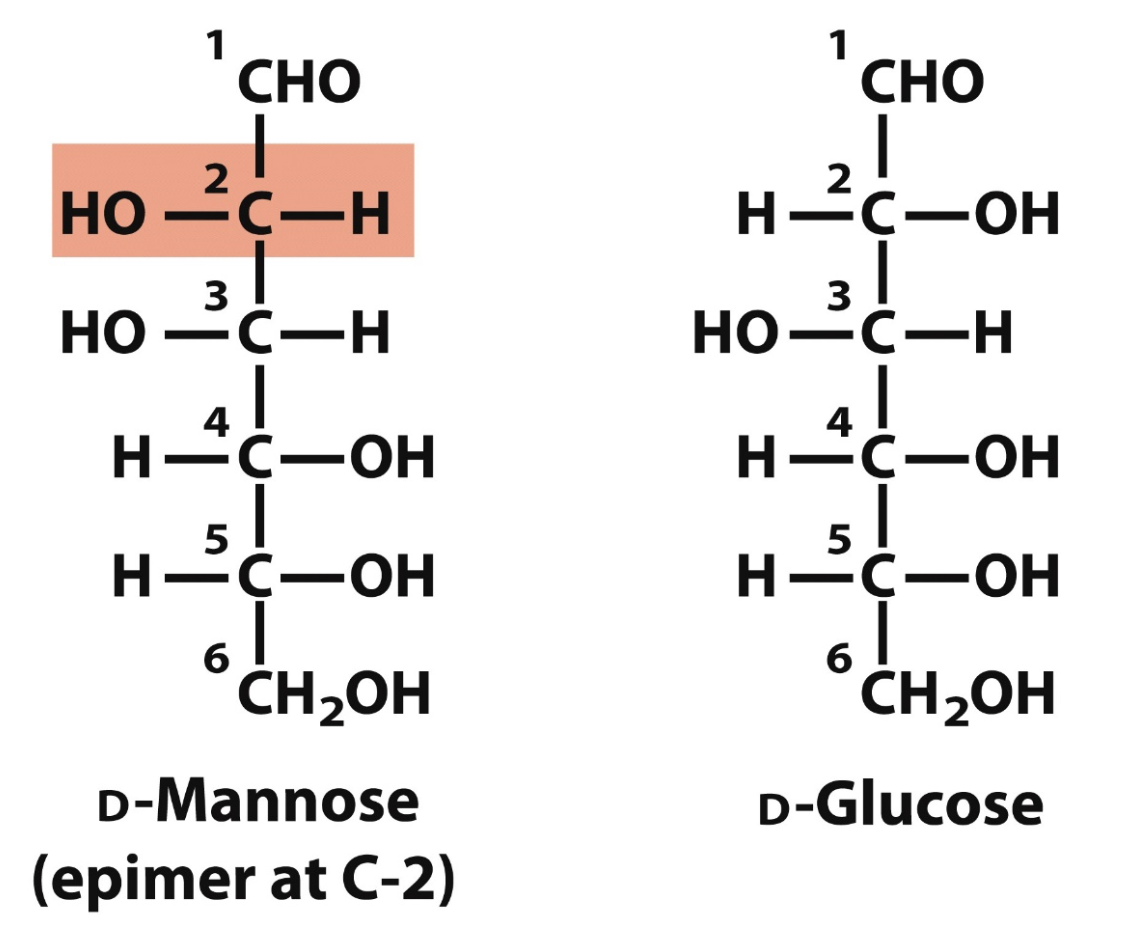
D-Glucose shape
ta too ta ta
ta too ta ta
right left right right (-OH)
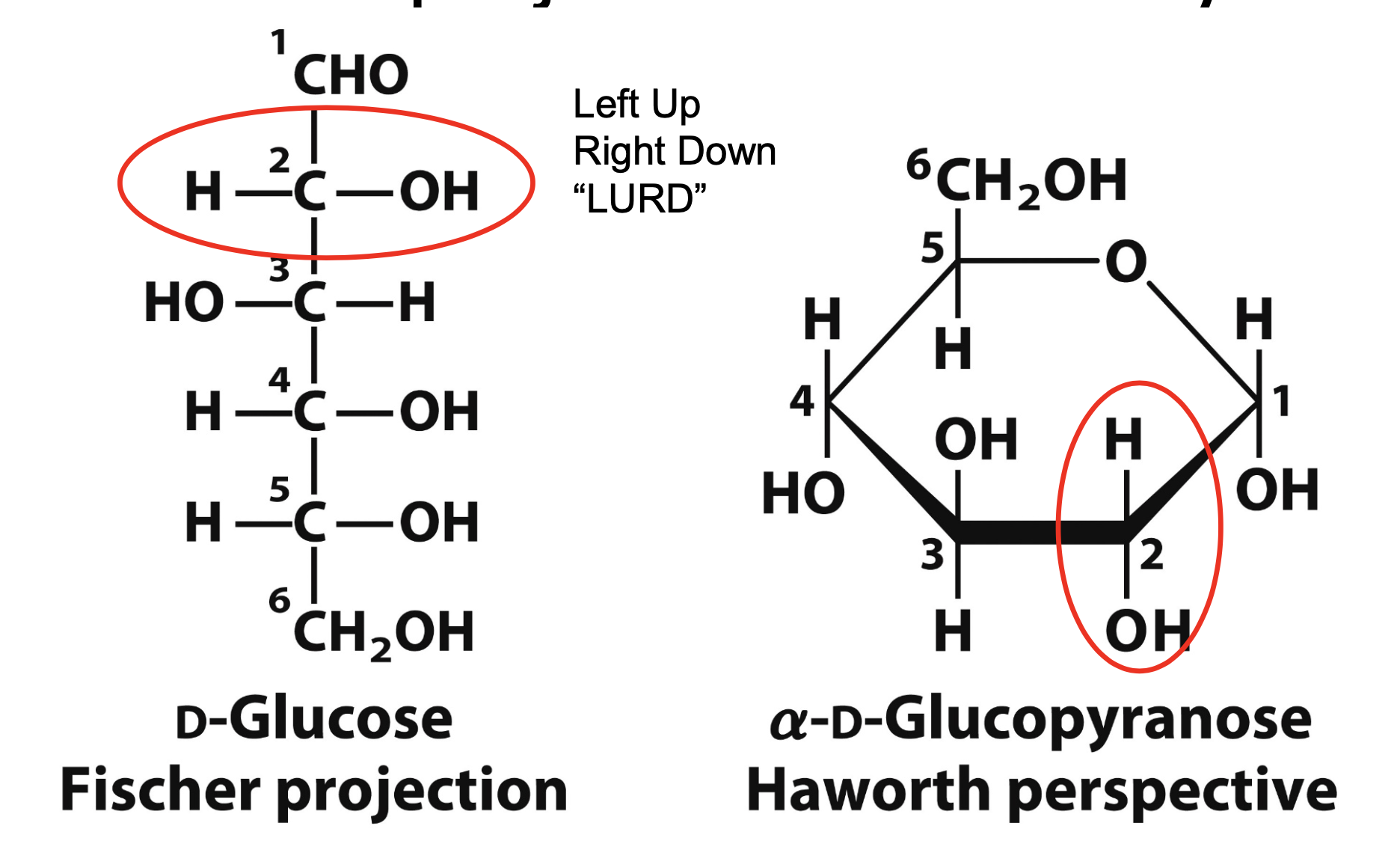
pyranose rings
Hexoses are not open chains
Open chains cyclize into rings
For an aldohexose like glucose the C-1 aldehyde reacts with the C-5 hydroxyl group to from an intramolecular hemiacetal
Results in a 6-membered ring called a pyranose ring
fisher vs haworth
LURD
chair vs boat
chair more stable
Acetyle group
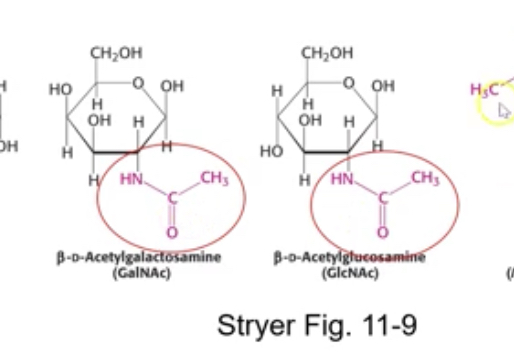
Formation of glycosidic bonds
reactive group is anameric group
C1, condemnation reaction, water created
Ether linkage
What are multiple monosaccharide joined together called?
Oligosaccharide or polysaccharide
sucrose
lactose
maltose linkage
sucrose - alpha-1,2
lactose - beta-1,4
maltose - alpha-1,4
naming glycosidic links
always involve C1 (hemiacetal) of first sugar
alpha or beta anomer of C1
alpha -OH down
beta -OH up
linked to what carbon on second sugar?
how many ways can two D-glucose units be linked?
11
trisaccharide - 176
sucrose
lactose
maltose
sucrose: glucose + fructose
lactose: galactose + glucose
maltose: glucose + glucose
homopolysaccharide
polysaccharide where repeating units are all the same
true or false: carbs are unbranched
false! they can be branched (fuel storage) or unbranched (structure)
branched vs unbranched carbs
branched = fuel storage
unbranched = structure
glycogen branching
alpha-1,4 chains of glucose
alpha-1.6 glycosidic link to create branch
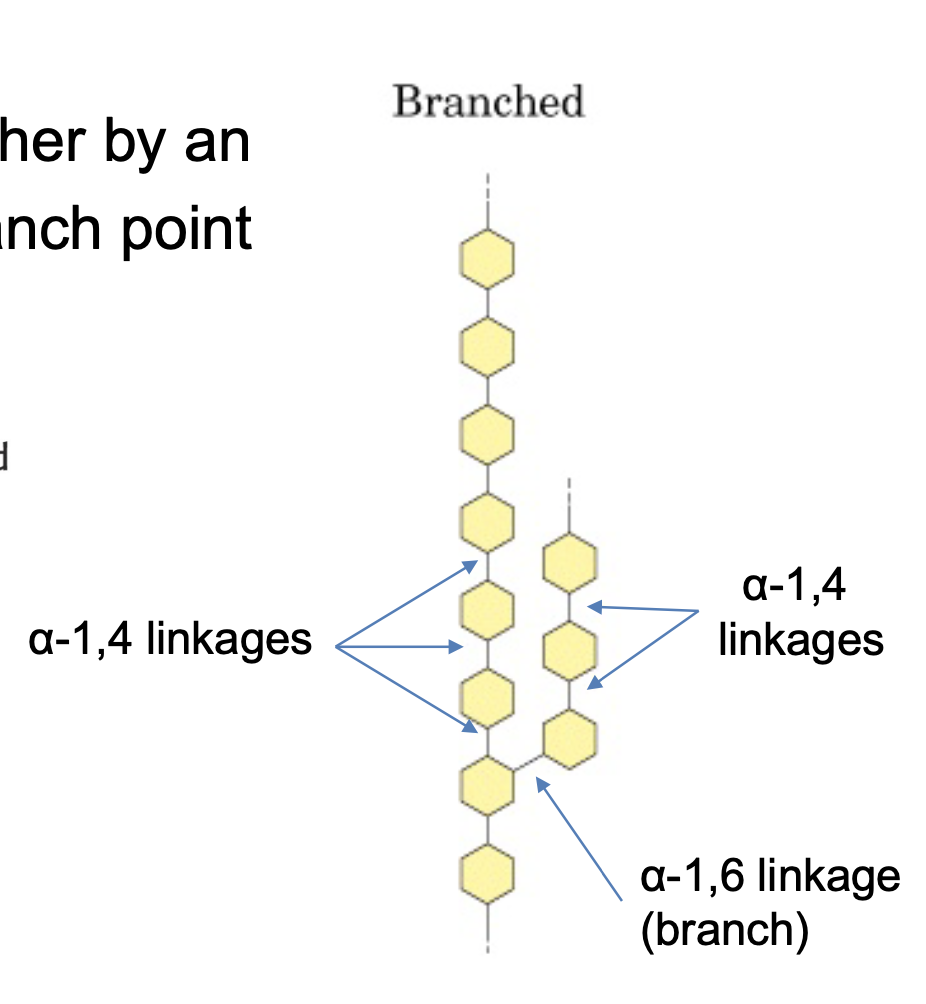
oligosaccharide ends
non-reducing vs reducing end
monosaccharide can be cyclic or linear straight chains
straight chain aldehyde can reduce other groups
oxidised to carboxylic acid
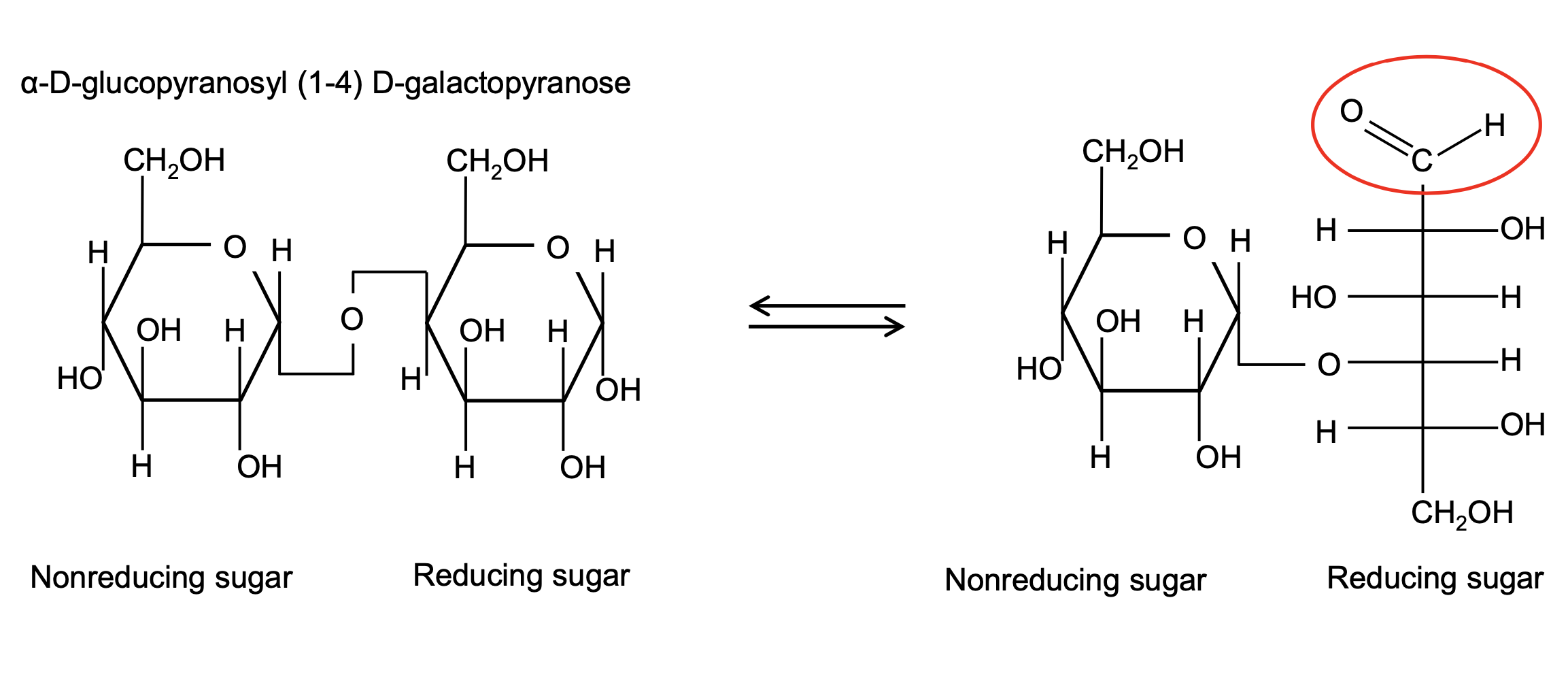
what affects structure of polysaccharides?
monosaccharides and nature or linkage