Electron geometry, Molecular Geometry, Molecular Geometries, Electron and Molecular Geometries
1/75
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
76 Terms
Resonance
When a molecule can have two or more equivalent structures, usually with different arrangement of double bonds
Linear
2 bonds, 0 lone pairs

Trigonal Planar
3 bonds, 0 lone pairs
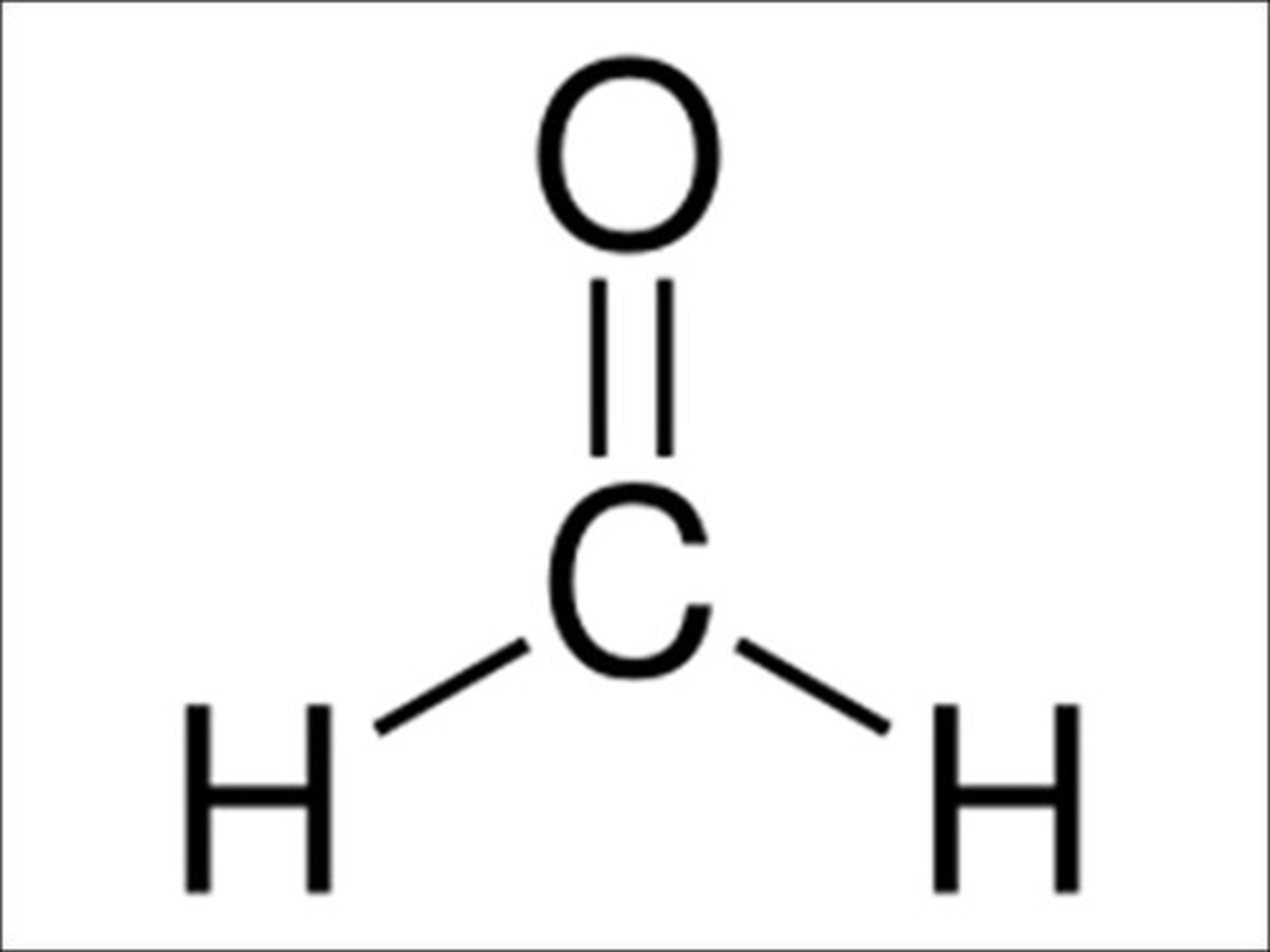
Bent
2 bonds, 1 lone pair

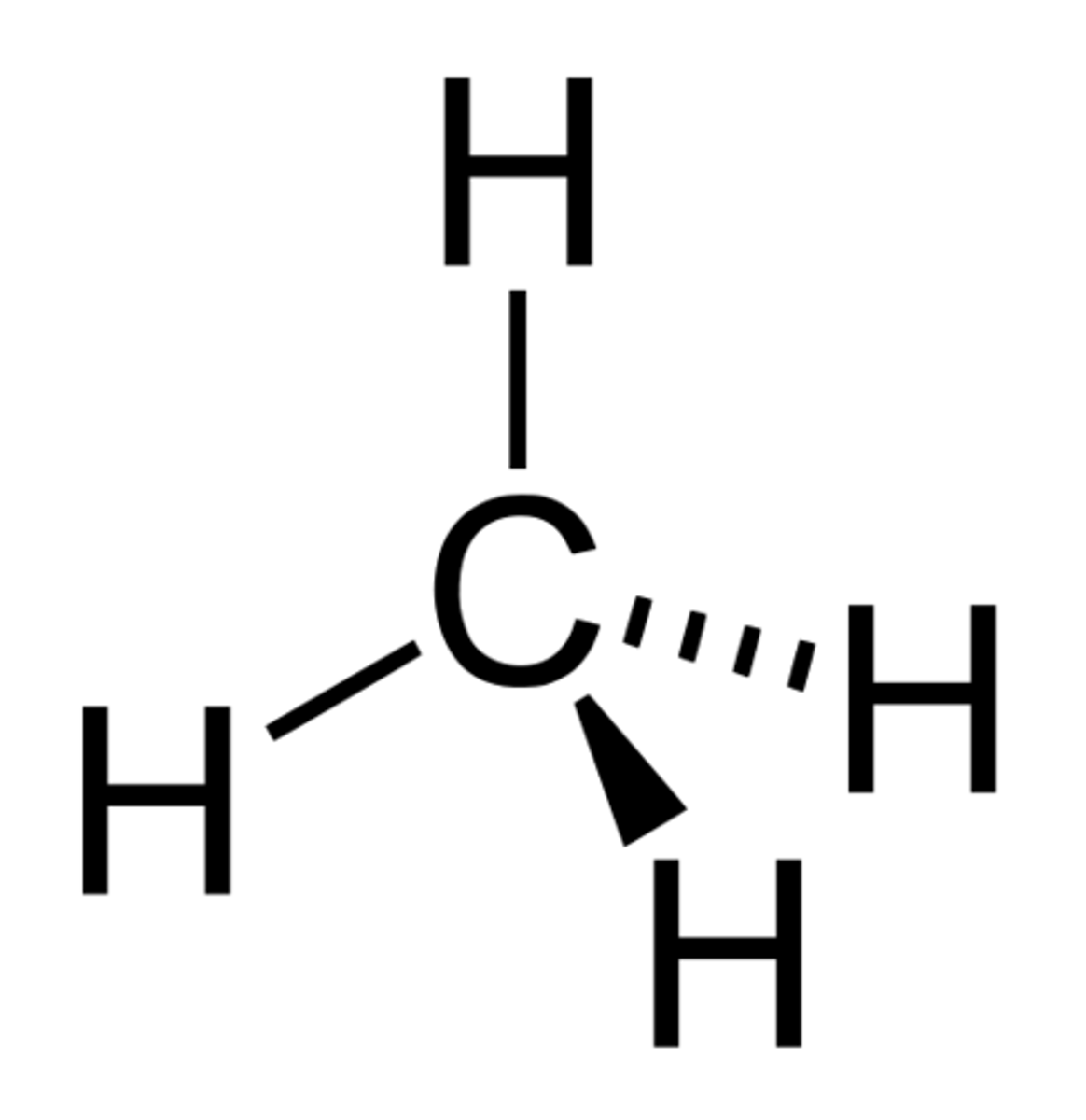
Tetrahedral
4 bonds, 0 lone pairs
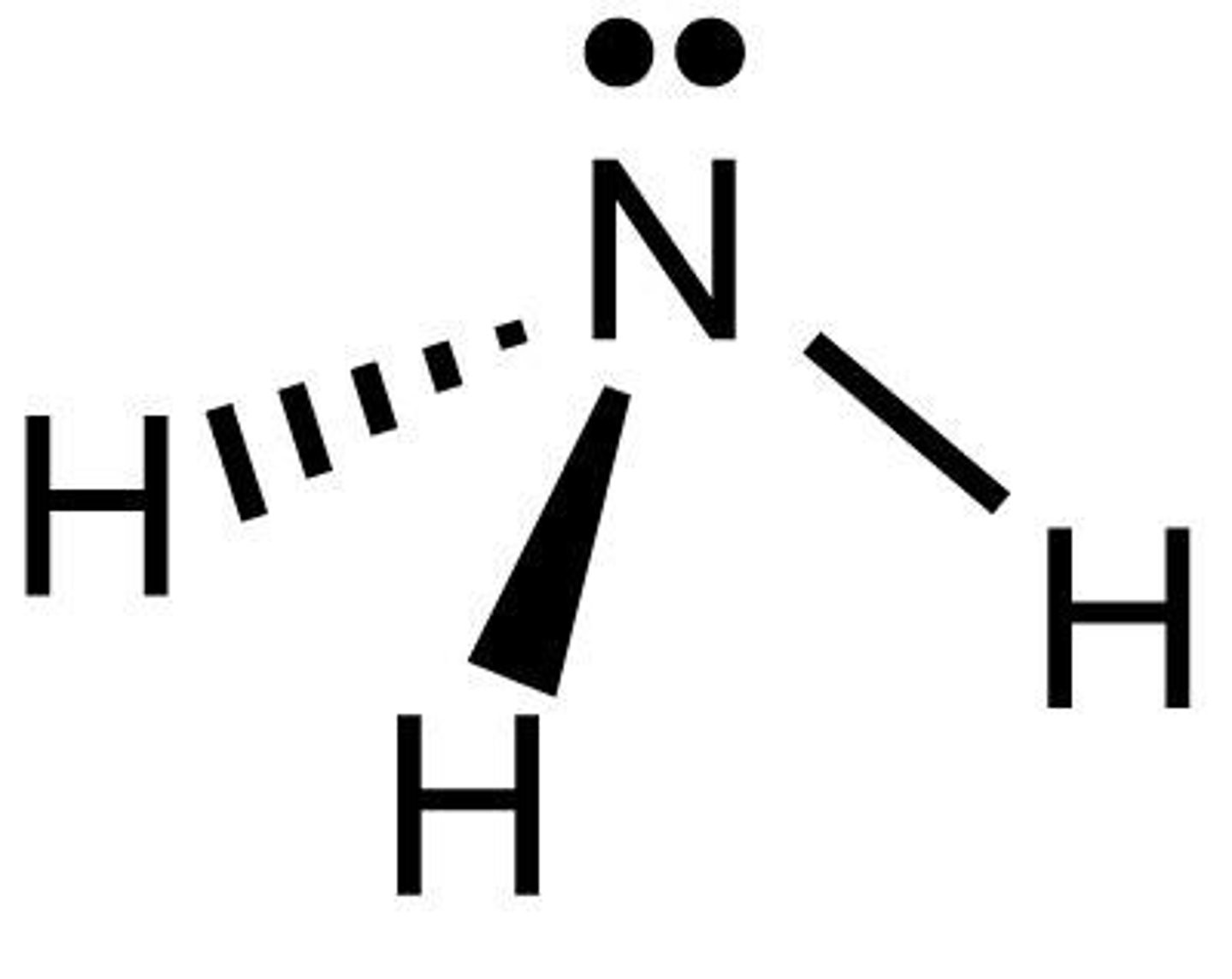
Trigonal Pyramidal
3 bonds, 1 lone pair
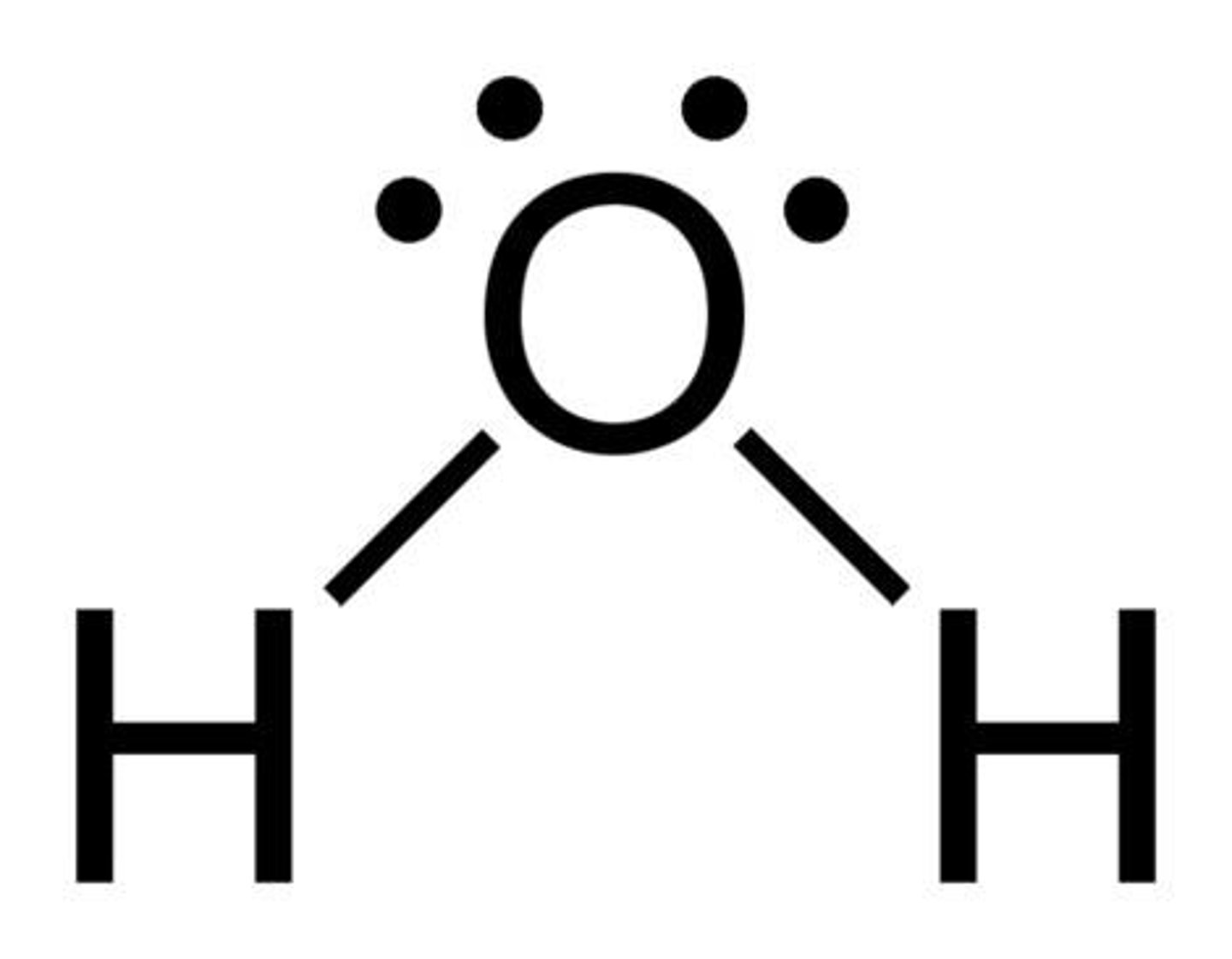
Bent (complex)
2 bonds, 2 lone pairs
Trigonal Bipyramidal
5 bonds, 0 lone pairs
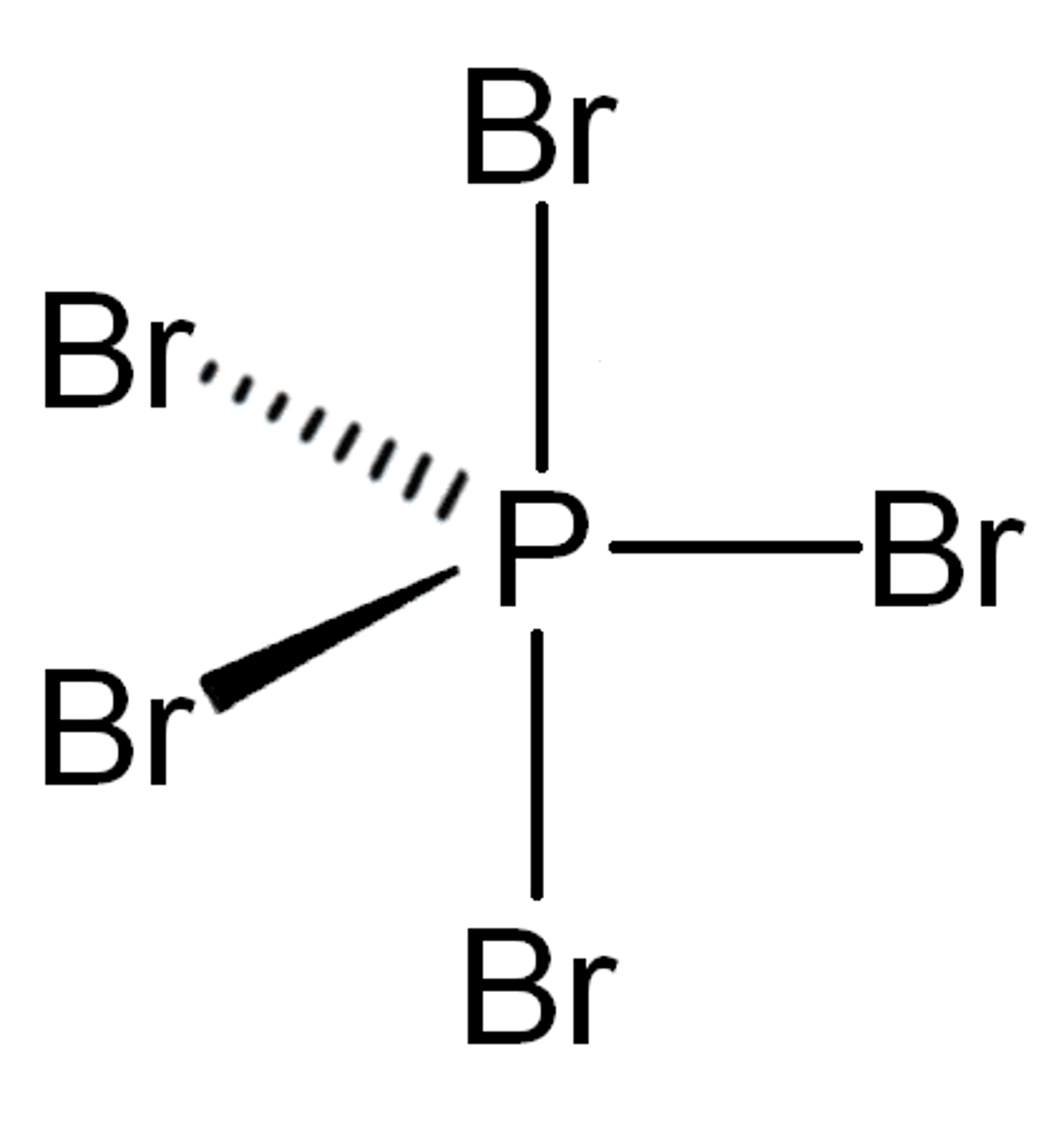

4 bonds, 1 lone pair
See-Saw

3 bonds, 2 lone pairs
T-shaped

2 bonds, 3 lone pairs
Linear (complex)
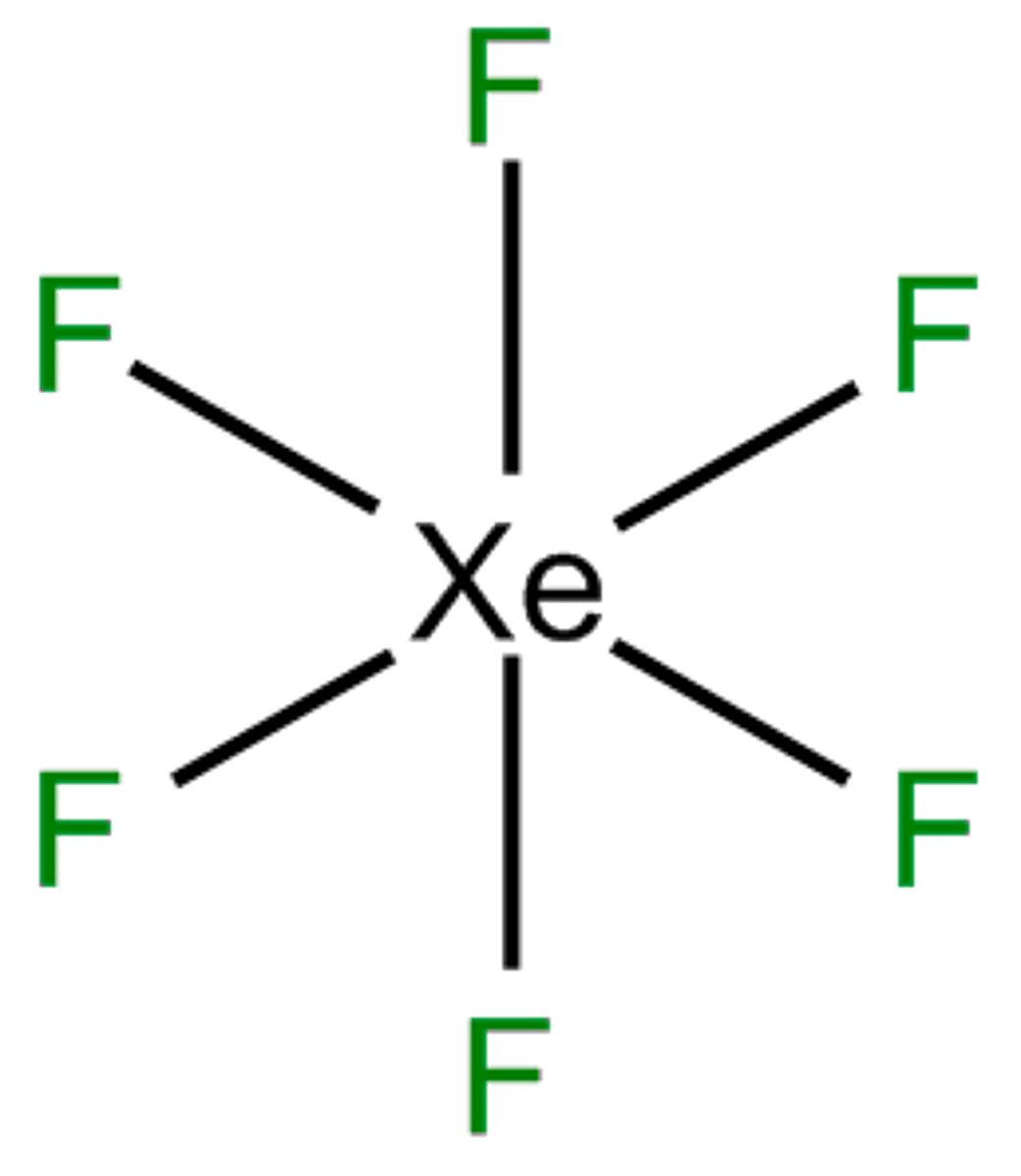
6 bonds, 0 lone pairs
Octahedral
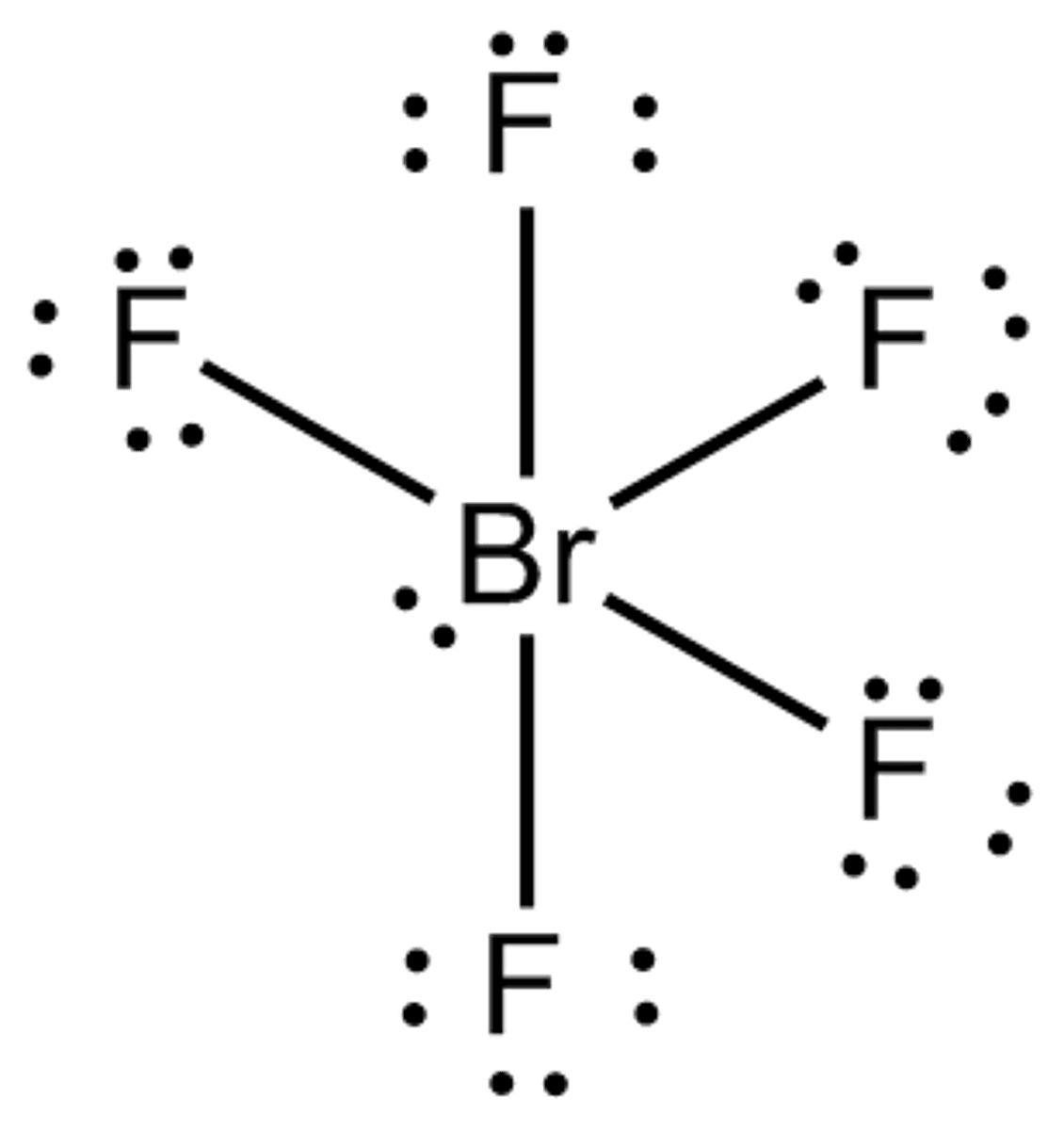
5 bonds, 1 lone pair
Square Pyramidal
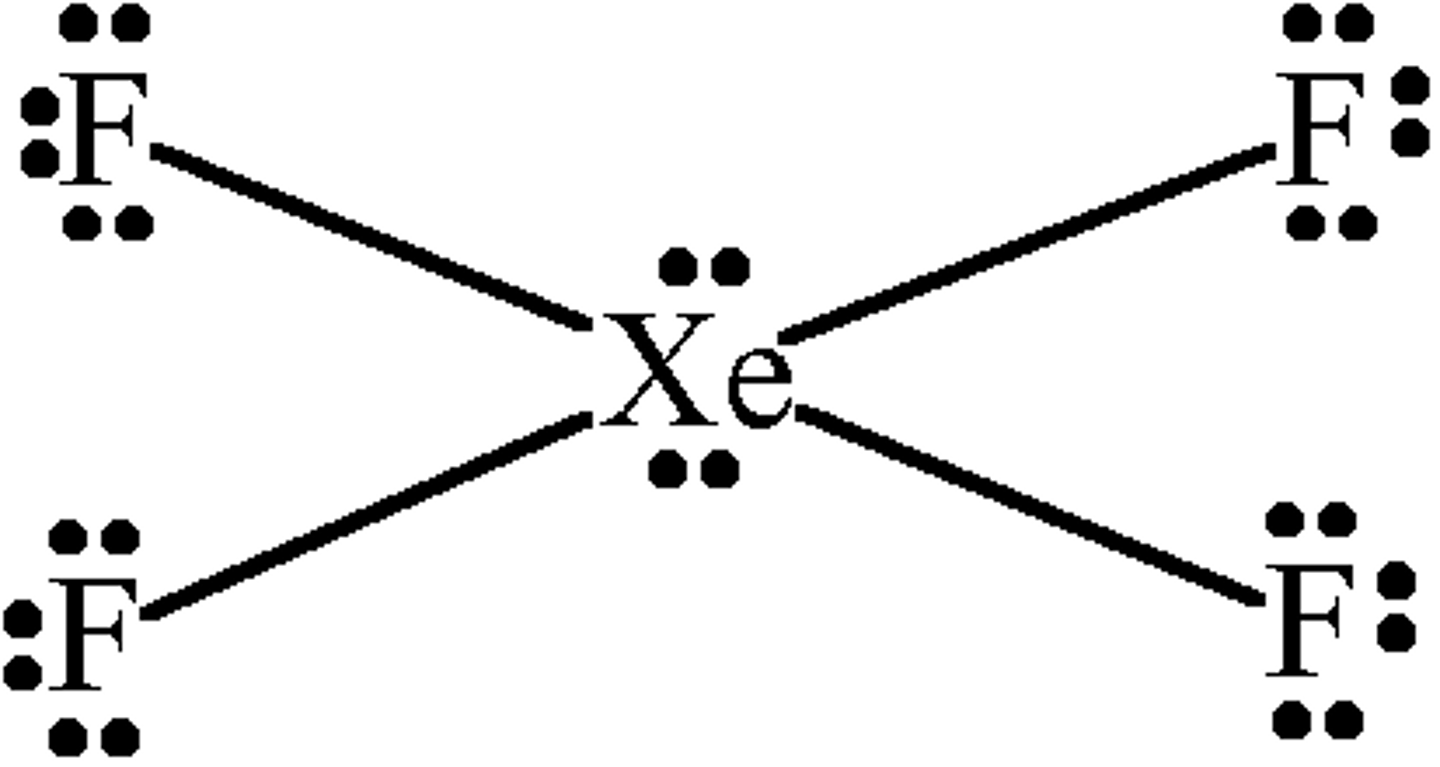
4 bonds, 2 lone pairs
Square Planar
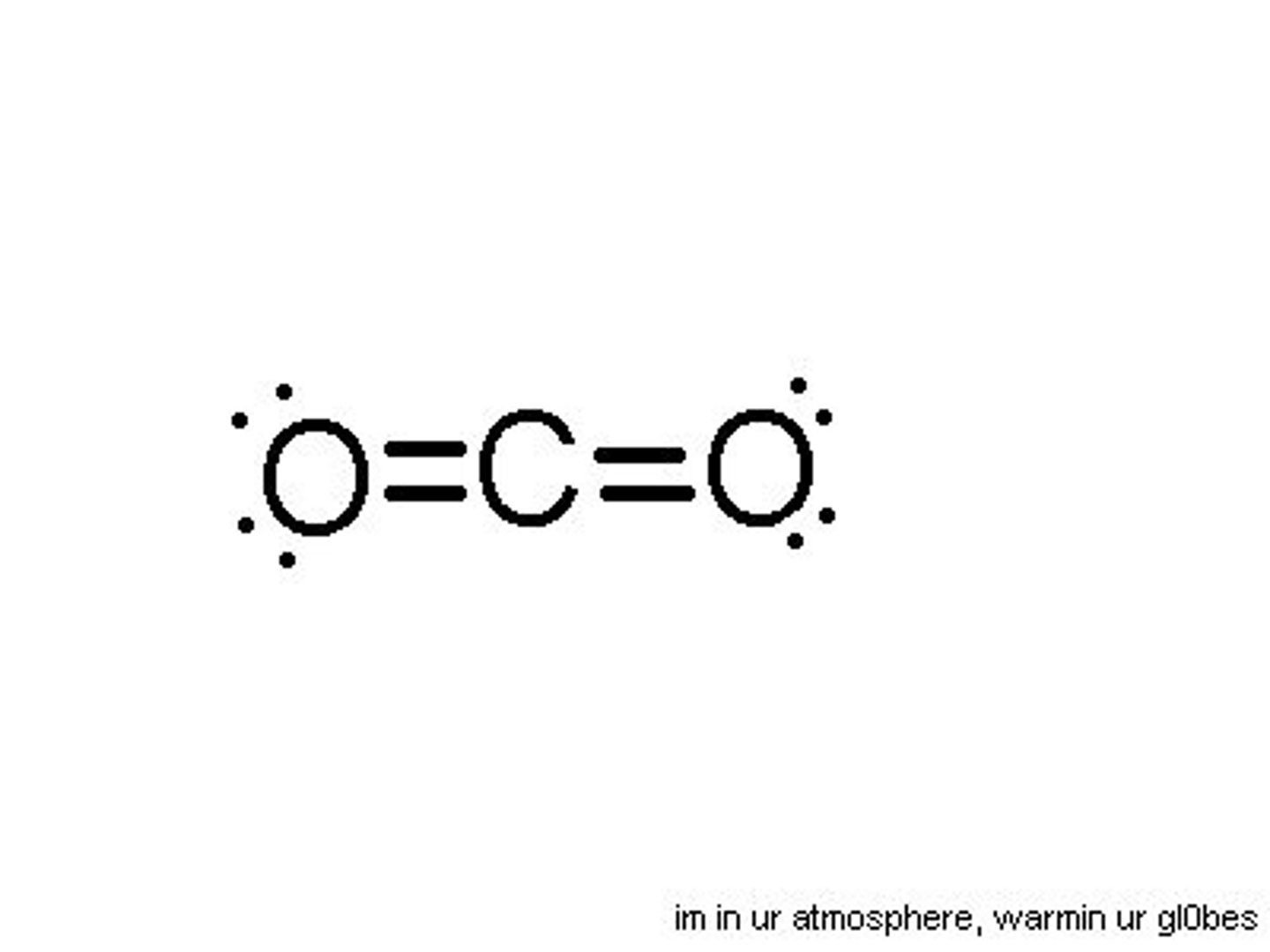
linear
180, sp
trigonal planar
120, sp2

tetrahedral
109.5, sp3
linear, 180
sp
trigonal planar, 120
sp^2
tetrahedral, 109.5
sp^3
trigonal bipyramidal, 90 and 120
sp^3d
Octahedral, 90
sp^3d^2

What is the moelcular geometry of this?
Linear
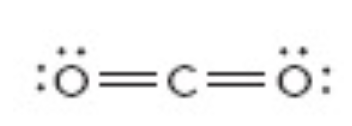
What is the electron geometry of this?
Linear
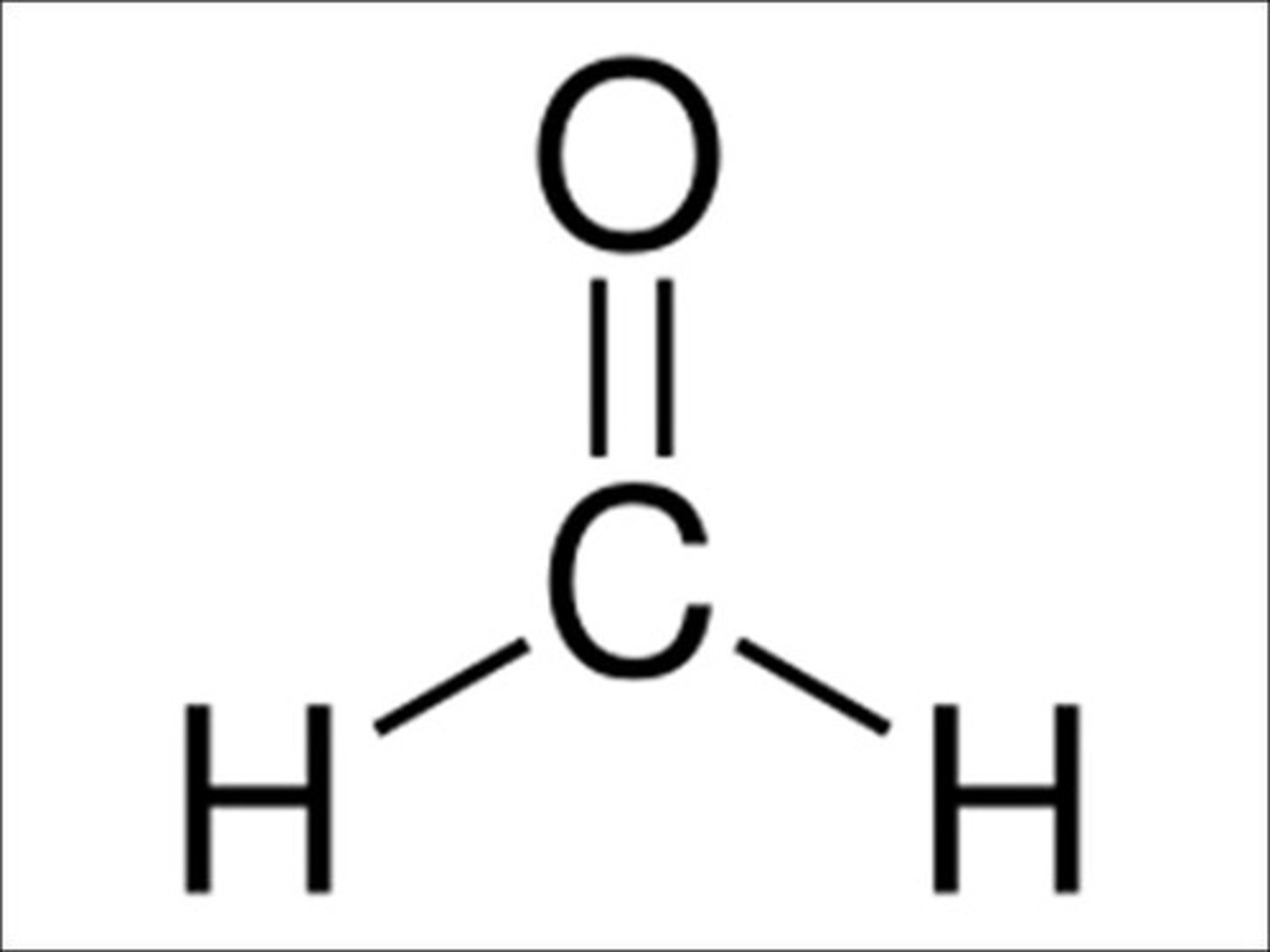
What is the moelcular geometry of this?
Trigonal planar
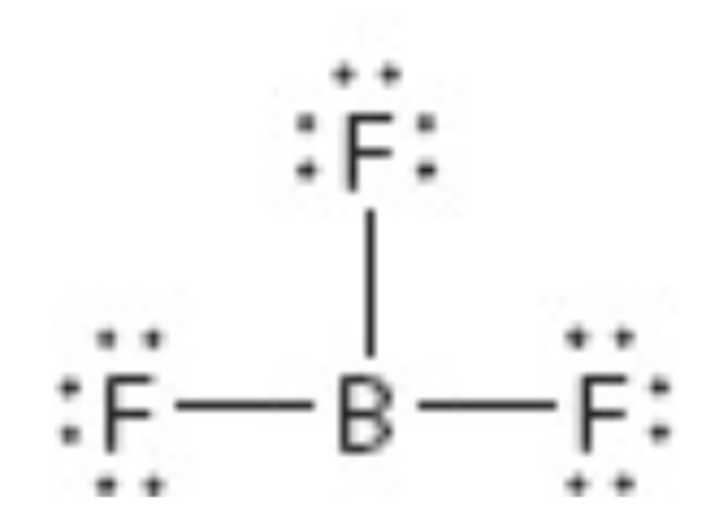
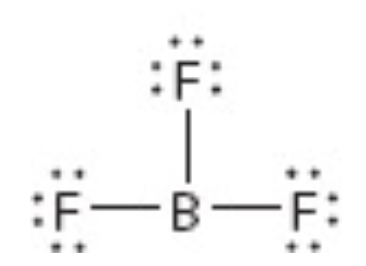
What is the electron geometry of this?
Trigonal planar
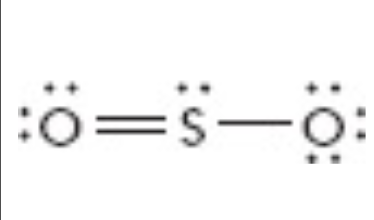
What is the moelcular geometry of this?
Bent
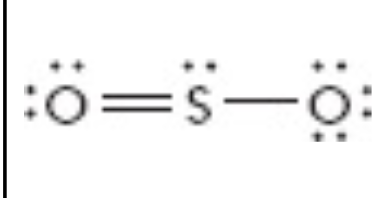
What is the electron geometry of this?
Trgional planar
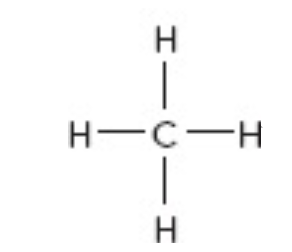
What is the moelcular geometry of this?
tetrahedral
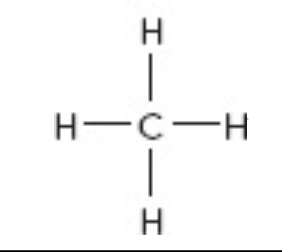
What is the electron geometry of this?
tetrahedral
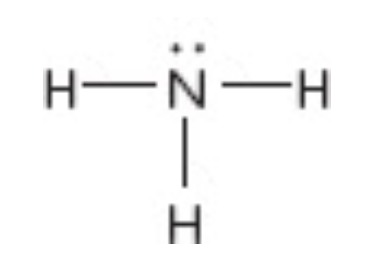
What is the moelcular geometry of this?
Trigonal pyrimidial
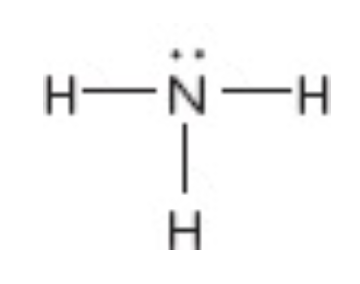
What is the electron geometry of this?
tetrahedral
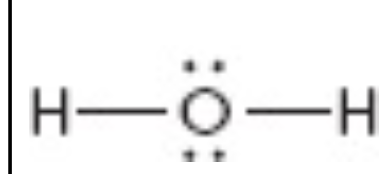
What is the electron geometry of this?
tetrahedral
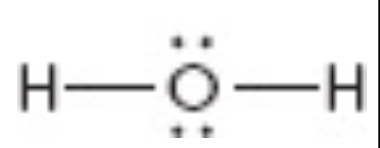
What is the molecular geometry of this?
bent
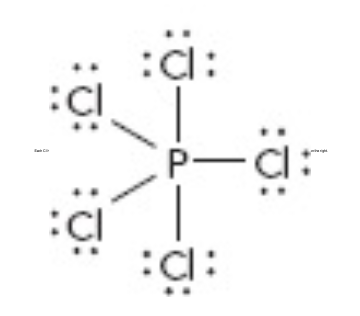
What is the electron geometry of this?
Trigonal Bipyramidal

What is the molecular geometry of this?
Trigonal Bipyramidal
What is the molecular geometry of this?
seesaw
What is the electron geometry of this?
trigonal bipyrimidial
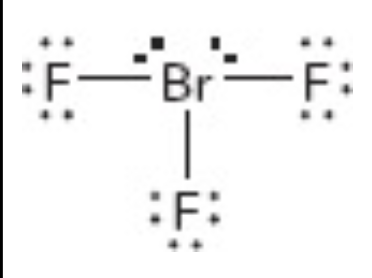
What is the electron geometry of this?
trigional bipyrimidial
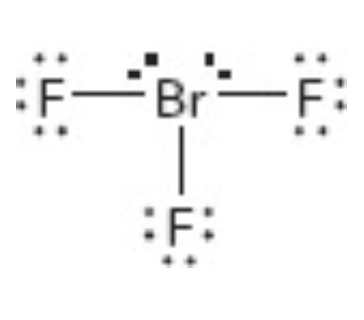
What is the molecular geometry of this?
t shaped
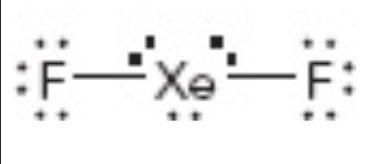
What is the molecular geometry of this?
linear
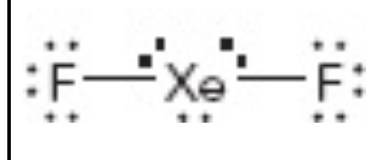
What is the electron geometry of this?
trigonal bipyrimidal
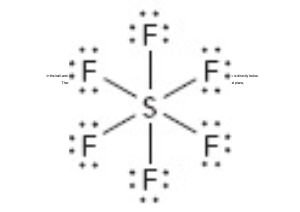
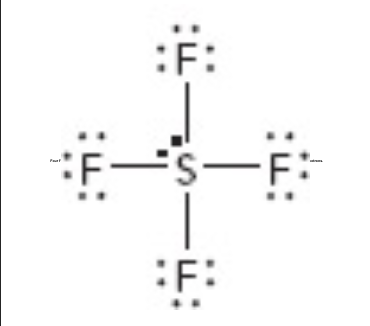
What is the molecular geometry of this?
octahedral
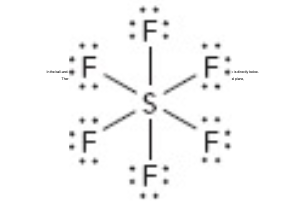
What is the electron geometry of this?
octahedral
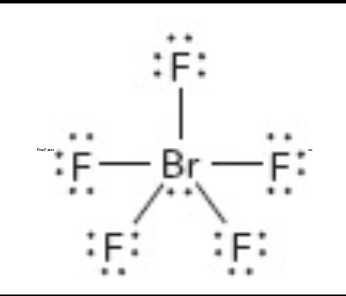
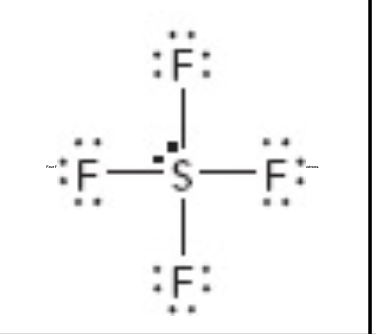
What is the molecular geometry of this?
square pyrimidial
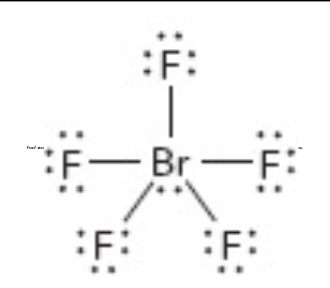
What is the electron geometry of this?
octahedral
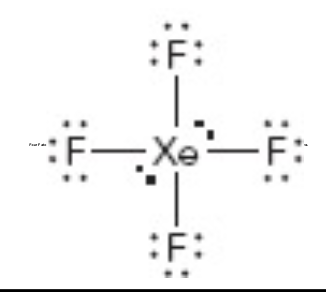
What is the molecular geometry of this?
Square planar

What is the electron geometry of this?
octahedral
Molecular geometry
when we do not distinguish between bonding electron groups and lone pairs
electron geometry
When we consider only the geometrical arrangement of the atoms
How can a molecule has polar bonds yet sill be nonpolar?
The bonding electrons are pulled equally toward both O ends of the molecule, while also taking in mind the molecular geometry of the moelcule. The net result is a nonpolar molecule
How can a bond be polar?
when there is a greater or lesser electronegativity in between a molecule
What are Hybrid orbitals?
regions of high probability of finding the shared electrons in the molecule
Why would some atoms hybridize?
to maximize bonding and therefore more full oribitals which would lead to more stability
Hybridizing
mixing different types of orbitals in the valence shell to make a new set of degenerate orbitals
According to valence bond theory, bonding takes place between atoms when their atomic or hybrid orbitals interact. To interact they must ____
be aligned along the axis between the atoms, or be parallel to each other and perpendicular to the interatomic axis.
What would be the hybridizatoon of an atom that has 5 electron groups?
sp3d
What would be the hybridizatoon of an atom that has 6 electron groups?
sp3d2
What would be the hybridizatoon of an atom that has 4 electron groups?
sp3
What would be the hybridizatoon of an atom that has 3 electron groups?
sp2
What would be the hybridizatoon of an atom that has 2 electron groups?
sp
What would be the hybridizatoon of an atom that has 1 electron groups?
s
What is a single bond in terms of sigma and pie bonds?
1 sigma
What is a double bond in terms of sigma and pie bonds?
1 sigma and 1 pie
What is a triple bond in terms of sigma and pie bonds?
1 sigma and 2 pie
Linear combination of atomic orbitals (LCAO)
atomic orbitals of the atoms adding together to make molecular orbitals
What are the two ways that ortibals can be added together using the Linear combination of atomic orbitals?
Constructively or Destructively
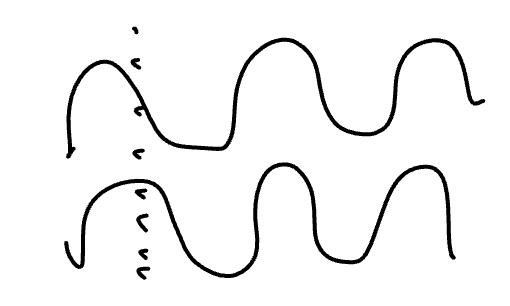
Would this be considered constructive or destructive adding of orbitals?
constructive
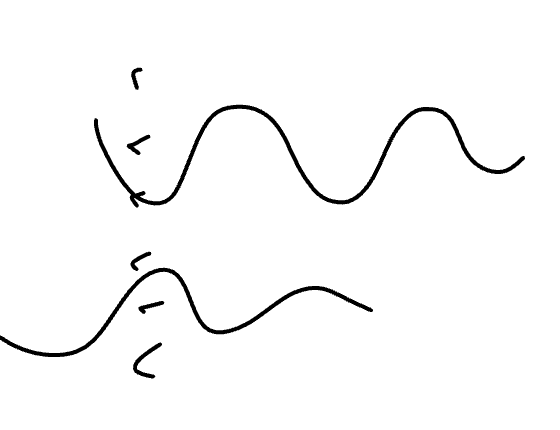
Would this be considered constructive or destructive adding of orbitals?
destructive
bonding molecular orbital
When the wave functions combine constructively, the resulting molecular orbital has less energy than the original atomic orbitals
antibonding molecular orbital
When the wave functions combine destructively, the resulting molecular orbital has more energy than the original atomic orbitals
Why are electrons in antiboding destabalizing?
Higher in energy than atomic orbitals
Electron density located outside the internuclear axis
Bond order
half the difference between number of electrons in bonding and antibonding orbitals
What is the formual of bond order?
# Bonding Electrons - # Antibonding Electrons/ 2
A substance will be _________ if its MO diagram has unpaired electrons
paramagnetic
A substance will be _________ if its MO diagram has paired electrons
diamagnetic