Week 3 - soil chemistry
1/35
Earn XP
Description and Tags
https://www.saskoer.ca/soilscience/chapter/soil-chemistry/
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
36 Terms
What are soil colloids? What does it do?
very small inorganic (clay) or organic (humus) particles
net negative charge in temperate soils
determines soil chemical properties
What type of exchange occurs with soil colloids?
the primary site for nutrient exchange in the soil + plant nutrient uptake
chemical cation/anion exchange, chemical reactions, adsorption of water
adsorption: molecules, atoms, ions gathering on surfaces
large surface area to help stick substances together
What is role of clay in soil?
4 types?
active mineral portion of soil
colloidal, crystalline, amorphous, morphous
What is clay in soil terminology?
<0.002 mm diameter particle
group of similar minerals
soil textural class
Why is clay important to humans and nature? 3
most important chemical weathering product of soil
many uses: construction, tiles, pottery
contribute to the exchange of ions
helps soil fertility in forestry + agriculture
What are different categories for determining clay type?
different material (silica, alumina) during formation
proportion of minerals + ions
amount of silica vs alumina
presence of ions
degree of acidity
pH of leaching (dissolution) water
How does the tropical biome affect the formation of clay?
hot, humid climate
highly leached soil
formation of different types of clay vs non leached soil
contains many dissolved primary minerals that recrystalize the clay that are different vs non leached soil
clay types:
kaolinite, allophane, smectite, sesquioxide
How does the temperate biome affect the formation of clay?
cold, moist-dry climate
leached-less leached soil
less dissolving and recrystallization
clay types:
vermiculite: clay formed from mica
similar properties to primary minerals (slight alteration)
selective/incomplete solubility
montmorillonite, illite, bentonite
What are the 3 sources of clay?
inherited clay
deposited as clay in sediment from another location
clay could be from different geologic period
modified clay
weathering/degradation of original clays
neoformed clay
new clay formed from leaching of primary materials
does not inherit any structure from a pre-existing mineral
What are the 2 crystalline natures of clay?
What are they bonded together by?
morphous clays: definite, regular, repeating arrangement of atoms
amorphous clays: irregular structure
morphous + amorphous clay:
composed of planes of oxygen bonded by silica + alumina (ionic bonding) or other ions
type of ion = type of clay
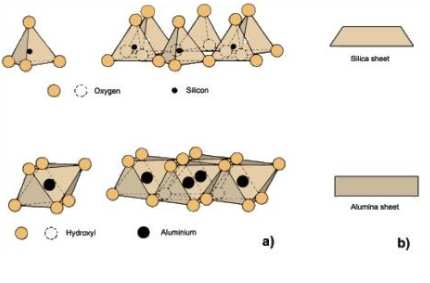
What are the types of silicate clays?
amorphous silicate clay: allophane, imogolite
morphous silicate clay - 1:1 silicate clay: kaolinite, halloysite
morphous silicate clays - 2:1 silicate clay: smectite clays, illite, vermiculite, chlorite
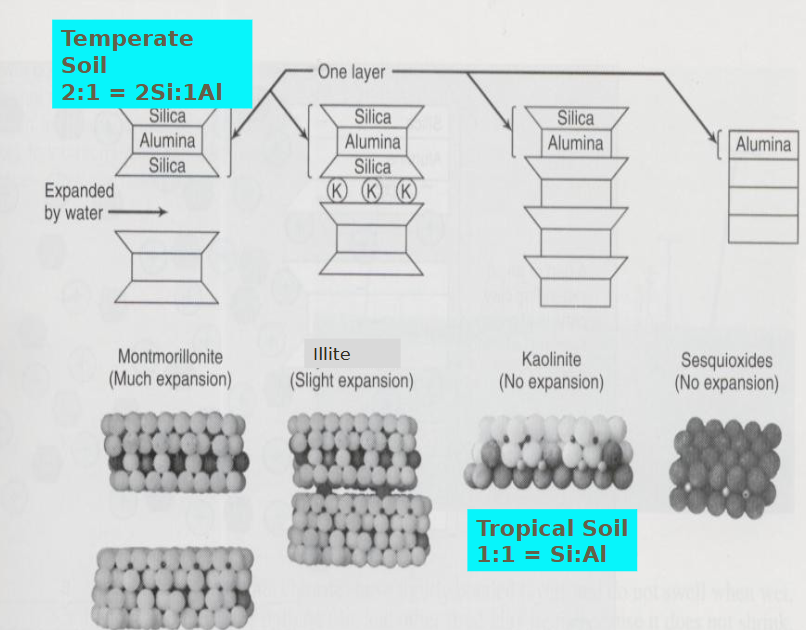
amorphous silicate clay: name clay, chemical properties, location, ionic bonds
allophane (most common) + imogolite
allophane = Al2O. 3· (SiO2)
mix of Al + Si = no well formed crystals + repetitive structure
highly weathered conditions w/ insufficient time to develop crystal growth
common in soils from volcanic ash
unusual = high affinity for P
morphous silicate clay – 1:1 silicate clays: name clay, chemical properties, location, ionic bonds
kaolinite (most common 1:1 clay) + halloysite
residues from extensive weathering
in high rainfall areas, typically leached, well drained + acidic soils
found in humid warm climate (florida, subtropics, tropics)
classified as kandite clays (for pottery)
does not allow water between layers
strong H bonding
kaolinite = non swelling clay
What clays are commonly used?
kaolinite
clay saturated with water → molded → hardened
non expanding nature = can fire dry without cracking
pottery, tiles, bricks
oxidation changes clay from grey to red
vermiculite
expands 20-30x size
insulation, plant potting material, packing, fireproofing
smectite: chemical properties, location, ionic bonds
montmorillonite, saponite, bentonite
swell when wet, shrink when dry
little or no leaching
dry soil
arid + semi arid regions (poorly drained)
develop from alkaline bedrock
illite: chemical properties, location, ionic bonds
k ions holding layers together
layers held tight + little water penetration between layers
slight to moderate swelling
found in soils not extensively weathered + high in primary minerals
occurs in similar environment as montmorillonite
vermiculite: chemical properties, location, ionic bonds
worm like structure
expands 20-30 times its original size
clay layers held together weakly
in warm/dry climate + well drained soil
chlorite: chemical properties, location, ionic bonds
classified as phyllosilicate clays
similar to vermiculite in structure
dominant cation = Mg
2 layers of silica, 1 layer of alumina, 1 bonded interlayer of Mg (2:1:1)
interlayer restricts swelling + has positive charge
common in soil formed from sedimentary rock
serpentine soils: green, brown or spotted minerals (in northern california)
non-silicate clays: name clay, chemical properties, location, ionic bonds
gibbsite + geothite
classified as sesquioxide clays
extensive leaching in tropics
yellow, red, brown soils
silicates washed away → leaving Al + Fe oxides remains
aluminum hydroxide, iron oxide + hydroxide
not sticky, does not swell
can absorb a lot of water, very stable in soil
high surface adsorption of P = less P for plants
What are organic colloids? Chemical properties?
stability, morphous, 2 types, CEC?
humus: intermediate product of highly decomposed plant and animal remains
most stable form of organic matter in soil (low solubility, low reactivity)
overall neg charge (like clay)
amorphous
separated by molecule size
fulvic acid + humic acid
higher cation exchange capacity vs clay
What do soil colloids do in cation exchange?
net neg charge that attract pos charge ions (cations) to the surface
cations in soil solution approach soil colloid → exchange of cations between soil solution + soil colloid = cation exchange
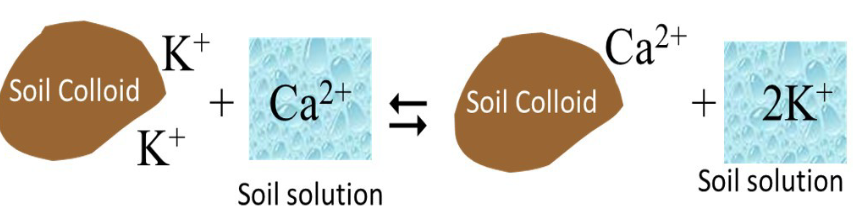
Where does cation exchange occur?
clay colloids, humus colloids, plant roots
Properties of absorbed cations
absorbed cation to soil colloid resist leaching (but can be removed by mass action)
competition for negative site by a large number of ions present in soil solution
strength of the adsorption depends on which ion is exchanged
Common cations
Ca2+, Mg2+, H+, Na+, K+
How to increase/decrease the proportion of cations on soil colloid surface?
addition by: dissolving minerals, liming, gypsum, fertilizers
losses by: plant adsorption, leaching
How is cation exchange mechanism affected by different ions?
ions move at different speeds
held with different forces of attraction to the exchange site
different soil types = influence the strength of ions held on to the exchange site
What does the strength of ion attraction depend on?
increasing valence (the combining power with other atoms when it forms chemical compounds/molecules)
cation’s hydrated size decreases
strength of site’s negative charge
Heavy metals and cation absorption strength
heavy meatals stay absorbed to the exchange sites
contaminated water percolating through soil becomes clean as contaminants are absorbed to the soil
can use microbes to clean the contaminants
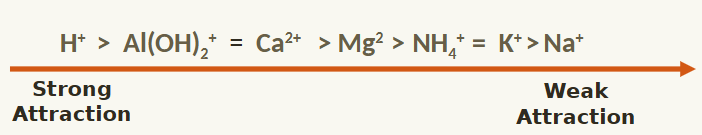
What is the cation exchange capacity CEC? When will it change?
CEC = measure of the quantiy of exchangeable cation sites per unit weight of dry soil
cmolc/kg or centimols of cations per kg of dry soil
depends on the type of soil
sands = 1-5 very small
clays = >30
inc in CEC = inc clay content in soil
will change when:
soil pH, humus content, clay content changes
Why is cation exchange important?
key for soil fertility
cause or correct soil acidity
alters soil physical properties
purifies percolating water (contaminants)
losses of nutrients via leaching
supplies nutrients to growing plants
hold fertilizer (K+ and NH4+)
What is anion exchange
anions = negatively charged nutrients
sulfate, nitrate, phosphate, molybdate, bobrate, chloride
not held on to cation exchange sites
The highest anion exchange capacity (AEC) occurs in soil with:
amorphous silicate clay (volcanic origin)
aluminium and iron hydrous oxide clays
to a lesser extend in kaolinite
mostly tropical and subtropical soil
strong weathering conditions, low pH
soils with high % of iron oxides
AEC vs CEC
AEC = 1/10 of centimole (1/10th of CEC’s size)
How strongly are anions held?
phosphates = strongly held to the soil (strong attraction)
common phenomena in tropical soils with low pH
strong attraction of phosphates to soil colloid = P unavailable for plants + dec in crop prod
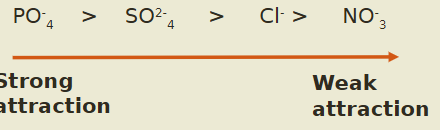
variable charge soil
CEC + AEC of soil = function of pH
soils with a variable chage
tropical/subtropical - acidification
arid zones – alkalinization
soil pH changes when SOM + mineral content are altered
pH rises = strong trend to CEC + weak trend to AEC (pH 5.0 to 5.2)
Basic vs acid soil and cations
acid soil – leaching of cations
areas of high rainfall
replacement of cations by H+
H+ replaced by Al(OH)2+
basic soil – no leaching
areas of low rainfall
soil high in Ca = basic
0 = acidic, 14 = basic