Carbohydrates and Lipids | Quizlet
1/51
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
52 Terms
Define covalent bond.
A covalent bond forms when two atoms share a pair of electrons.
State why covalent bonds are essential in biological molecules.
They are the strongest bonds in biological systems, providing stability to DNA, proteins, and other molecules.
Outline how carbon's electron configuration contributes to molecular diversity.
Carbon has four electrons in its outer shell and can form four covalent bonds, enabling a vast array of molecular structures.
Describe the types of structures carbon can form.
Carbon can form straight chains, branched chains, rings, and multiple bonds (single, double, triple).
Define functional group.
Functional groups are clusters of atoms that determine a molecule's properties and reactivity.
Identify the functional group found in sugars and alcohols.
Hydroxyl group (-OH).
Identify the functional group found in amino acids and fatty acids.
Carboxyl group (-COOH).
Identify the functional group found in proteins.
Proteins contain two main functional groups in their amino acid monomers:
Amino group (–NH₂)
Carboxyl group (–COOH)
Define macromolecule.
A large molecule composed of smaller subunits called monomers.
Explain condensation reaction.
A reaction that joins monomers by removing water, forming covalent bonds.
Define hydrolysis.
A chemical reaction that adds water to break bonds between monomers.
State the difference between condensation and hydrolysis.
Condensation builds polymers by removing water; hydrolysis breaks them by adding water.
Define monosaccharide.
A simple sugar that cannot be hydrolyzed into smaller carbohydrates.
Give examples of pentoses.
Ribose and deoxyribose.
Give an example of a hexose.
Glucose – the main energy source for cells
Fructose – found in fruits and honey
Galactose – found in milk and dairy products
Mannose – involved in glycoprotein formation
Differentiate alpha and beta glucose.
Alpha has the -OH group on carbon 1 below the ring; beta has it above.
State the function of glucose.
It serves as a primary energy source in cells.
Explain how glucose's structure helps its function.
Small, soluble, chemically stable, easily transported for respiration.
State the two forms of starch.
Amylose (unbranched) and amylopectin (branched).
Compare amylose and amylopectin.
Amylose is unbranched and coils; amylopectin is branched and allows faster glucose release.
Describe glycogen's structure.
Highly branched alpha-glucose polymer, more branched than amylopectin.
Explain why glycogen is ideal for animal energy storage.
Glycogen is ideal for animal energy storage because it is highly branched, allowing rapid glucose release, compact for efficient storage, and insoluble, preventing water imbalance in cells. It is also easily broken down when energy is needed quickly.
Describe cellulose's structure.
Polymer of beta-glucose with 1→4 bonds and alternating orientation.
Explain why humans cannot digest cellulose.
Humans lack cellulase to hydrolyze beta-1→4 glycosidic bonds.
Define lipid.
A lipid is a nonpolar, hydrophobic organic molecule, meaning is made mostly of carbon, but also hydrogen, and some oxygen, that is insoluble in water but soluble in organic solvents.
Lipids include fats, oils, phospholipids, and steroids.
They serve key roles in energy storage, cell membrane structure, and hormone production.
Define triglyceride.
A triglyceride is a type of lipid formed by the condensation reaction of one glycerol molecule and three fatty acid molecules. It serves as a major form of long-term energy storage in animals and plants and is also important for insulation and protection of organs.
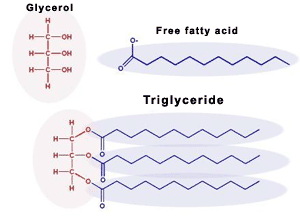
Explain why lipids are hydrophobic.
They have long non-polar hydrocarbon chains that repel water.
Describe a condensation reaction forming triglyceride.
Three fatty acids form ester bonds with glycerol, releasing three water molecules.
List the biological roles of triglycerides.
Energy storage, insulation, cushioning organs.
Define fatty acid.
A long hydrocarbon chain with a terminal carboxyl group.
Differentiate saturated and unsaturated fatty acids.
Saturated have no double bonds; unsaturated have one or more.
Describe how double bonds affect fatty acid structure.
They introduce kinks, preventing tight packing and lowering melting points.
State functions of phospholipids.
Form cell membranes, provide structural integrity, and enable selective permeability.
Explain the amphipathic nature of phospholipids.
They have hydrophilic heads and hydrophobic tails.
Describe the structure of a steroid.
Four fused carbon rings (three cyclohexane, one cyclopentane).
Give an example of a steroid hormone.
Testosterone or oestradiol.
Explain how steroids enter cells.
Being non-polar, they diffuse directly through the phospholipid bilayer.
Define glycoprotein.
A protein with carbohydrate chains for cell recognition and signaling.
State a function of glycoproteins.
Immune recognition and determining blood types (e.g., ABO system).
Compare energy yield of lipids and carbohydrates.
Lipids yield ~9 kcal/g, carbohydrates ~4 kcal/g.
Explain why triglycerides are suited for long-term energy storage.
High energy density, hydrophobic, compact.
State why polysaccharides do not cause osmotic problems.
They are insoluble due to their size.
Explain why lipids are better for insulation than carbohydrates.
Lipids are poor conductors of heat and form thick layers.
Compare the functions of starch and cellulose.
Starch stores energy; cellulose provides structural support.
List the four major classes of carbon compounds used by living organisms.
Carbohydrates, lipids, proteins, and nucleic acids.
List example molecules with branched chain, unbranched chain, single ring or multiple rings.
. Branched Chain Molecules:
Glycogen – highly branched polymer of α-glucose (ideal for rapid energy release in animals)
Amylopectin – moderately branched form of starch (found in plants)
2. Unbranched (Straight) Chain Molecules:
Amylose – unbranched, helical polymer of α-glucose (component of plant starch)
3. SIngle Ring Molecule
Sucose – a hexose sugar with a single ring in its cyclic form
Pyridine – nitrogen-containing single ring
4. Multiple Ring Molecules:
Cholesterol – steroid with four fused rings
Estrogen/Testosterone – hormones with multiple fused rings
Caffeine – purine structure with multiple rings
DNA bases (e.g., adenine, guanine) – double-ring nitrogenous bases
Purines - nitrogen-containing double ring
State that energy from ATP is needed to produce macromolecules by condensation reactions.
ATP provides energy to form covalent bonds during condensation reactions that build polymers.
Compare the structure of A, B and O glycoproteins on the red blood cell membrane.
A and B have different terminal sugars; O lacks the added sugar, affecting immune recognition.
Discuss the consequences of the presence of A, B and O glycoproteins during blood transfusion.
Mismatched transfusions can trigger immune responses due to antigen-antibody incompatibility.
Distinguish between the structure and properties of cis- and trans-unsaturated fatty acids.
Cis has hydrogen on same side of double bond, creating kinks; trans has hydrogens on opposite sides, leading to straighter chains and health concerns.
Draw a simplified diagram of the structure of the phospholipid, including a phosphate-glycerol head and two fatty acid tails.
A phospholipid consists of a hydrophilic phosphate-glycerol head and two hydrophobic fatty acid tails.
Identify steroid molecules from molecular diagrams.
Look for four fused rings: three hexagons and one pentagon; e.g., cholesterol, testosterone.