Chem H Bonding Quiz - van ness - smchs
1/8
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
9 Terms
Which of the following compounds contains an ionic bond?
O2, SO2, NaCl, NH3, CCl4
NaCl
Which of the following compounds contains 1+ covalent bonds?
NaBr, MgS, MgBr2, SO2, Rb2O
SO2
In ionic bonding ____ is true.
a noble gas configuration is formed for each element or ion,
the electrons are shared between the atoms,
the process of forming an ionic bond is highly endothermic overall,
the bonding that occurs is usually between nonmetal atoms
a noble gas configuration is formed for each element or ion,
the bonding that occurs is usually between nonmetal atoms
An oxygen atom needs to gain _____ electrons to achieve a noble gas configuration.
2
The Lewis structure for which of the following contains the greatest number of lone pairs of electrons?
H2O, H2, F2, CH4, HF
F2
How many of the following will have Lewis structures with multiple bonds?
F2, CO2, H2O, N2, O2
3
The shape of ammonia (NH3) molecule is ….
trigonal pyramidal
Which of the following has a double bond?
H2O, H2S, C2H2, C2H4
C2H4
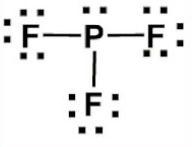
Draw the lewis structure for PF3
trigonal pyramidal