Lecture 6 - protein structure (beta sheets)
1/48
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
49 Terms
What is β-conformation or b-pleated sheet
It’s a secondary structure in which polypeptide chains align side-by-side, stabilized by hydrogen bonds between different strands.
key features b sheets? (4)
Peptide bonds are planar
H-bonds form between residues in adjacent strands
R groups are above and below plane of sheet
Amino acid preferences are highly context-dependent and generalizations are unhelpful and misleading
why are peptide bonds planar?
Like in α-helices, each peptide bond in a β-sheet is flat and rigid due to resonance.
This allows the chains to lay flat and align neatly in sheets.
what direction can strands lie and how does it affect hydrogen bonding?
Hydrogen bonds occur between two or more adjacent strands of the polypeptide. (not within the same strand like in α-helices).
The strands can be:
Antiparallel (opposite directions): more stable and straight H-bonds. (opposite N→C direction)
Parallel (same direction): slightly distorted H-bonds.
why do R groups alternate above and below the plane
This reduces steric hindrance and allows for tight packing.
Think of the backbone as a zig-zag ribbon, with side chains sticking out above and below like flags.
why is aa usually context dependent and generalization is misleading?
While we might say that certain amino acids "prefer" β-sheets (like valine or isoleucine), in reality:
Context matters more—surrounding residues, environment, and tertiary structure influence whether a residue will be in a β-sheet.
No amino acid is strictly limited to β-sheet or α-helix—it depends on the specific protein and its folding needs.
basically, Don't memorize "helix-formers" or "sheet-formers" in isolation—real protein folding is far more nuanced, and structural context determines what forms where.
what is common protein with a high content of b pleated sheets?
Fibroin, the main protein in silk, is rich in β-sheets.
Its amino acid composition:
Glycine 45% – very small, fits into tight spaces.
Alanine 29% – small and hydrophobic, stabilizes sheets.
Serine 12%
The rest: 1–2% other amino acids.
This sequence allows tight packing and extensive hydrogen bonding between strands, making silk strong yet flexible.
key characteristic of a-keratin?
α-keratin (found in wool and hair) is normally α-helical.
If you steam and stretch it:
The α-helices can be temporarily unwound and converted to β-sheets.
This is used in hair treatments (e.g., hair straightening or curling).
However, this change is not permanent—proteins can revert due to their thermodynamically preferred conformation.
what is The Science Behind Hair Curling?
Hair contains α-keratin, a protein rich in the amino acid cysteine. Cysteine side chains can form disulfide bonds (–S–S–), which:
Cross-link protein strands
Lock in the shape of hair (straight or curly)
explain the Hair Perm Process:
Heat + Reducing Agent
Example: Thioglycolic acid (structure shown in red)
It breaks disulfide bonds, turning –S–S– into –SH groups
Hair becomes flexible and can be reshaped
Reform Bonds in New Shape
Hair is wrapped around rollers or styled
An oxidizing agent like hydrogen peroxide (H₂O₂) is added
This reforms disulfide bonds between new cysteine pairs, locking the new shape in place
what is collagen?
collagen, a fibrous structural protein that’s unique because it does not contain α-helices or β-sheets—instead, it forms a left-handed helix called the polyproline helix.
what is collagens structure? (3)
Collagen makes up about 1/3 of the protein in the human body.
Found in skin, bone, cartilage, tendon, and teeth.
collagen structure is a right handed super helix which is made by 3 left handed chains called alpha chains?
is collagens Amino Acid Composition (very specific)?
Glycine (Gly) – 35% → tiny side chain fits in the tight central space of the helix.
Alanine (Ala) – 11%
Proline (Pro) – 12% → rigid, enforces helical twist
Hydroxyproline – 9% → a post-translationally modified form of proline.
what is Hydroxyproline?
Not one of the 20 standard amino acids.
Made by the enzyme prolyl hydroxylase, which converts Pro → Hydroxyproline.
enzyme Requires vitamin C (ascorbate) as a cofactor.
Lack of vitamin C → defective collagen → scurvy.
modified amino acid that plays a critical role in stabilizing the structure of collagen.
what is collagens structure (Polyproline Helix) built from?
Built from:
Three left-handed helices called α-chains
⚠ These are not the same as α-helices!Each α-chain has no internal hydrogen bonds (unlike α-helices or β-sheets).
Why?
Because proline and hydroxyproline dominate the sequence, and proline lacks a backbone N–H group (needed for donating hydrogen bonds).
The geometry of the left-handed helix doesn’t favor intra-chain H-bonds.
what is it stabilized by?
Steric repulsion between proline and hydroxyproline side chains, which keeps each chain extended and rigid.
The three α-chains twist together in a right-handed triple helix.,
The hydrogen bonds form between the chains to stabilize the right-handed triple helix.
Most of these bonds come from:
Hydroxyproline’s –OH group acting as a hydrogen bond donor
Backbone carbonyl oxygens from neighboring chains acting as acceptors
why is Glycine is Essential for collagens structure? (3)
The interior of the triple helix is extremely tight.
Only glycine, with its tiny –H side chain, can fit in the center.
Every third residue in collagen is glycine.
where does collagens strength come from?
Collagen’s strength and structure come from its tight triple helix, high glycine content, and inter-chain hydrogen bonding, not from α-helices or β-sheets.
What is Tertiary Structure
It’s the overall 3D folding of a single polypeptide chain, including how secondary structures (like α-helices and β-sheets) are arranged in space.
This folding creates a globular (compact) protein shape.
Hydrophobic vs. Hydrophilic R-Groups:
~40% of amino acids in a typical protein are hydrophobic (non-polar).
These are buried inside the protein core to avoid contact with water.
Hydrophilic (polar/charged) R-groups are found on the surface, where they can form favorable interactions with water (e.g., hydrogen bonds or ionic interactions).
what is are some things that provide stability to tertiary structure? (5)
Hydrophobic affect (main one)
disulfide bonds
electrostatic or ionic interactions
H-bonds
metal chelation
how does the hydrophobic help stability
Key Stabilizing Force: Hydrophobic Effect
The main driving force for folding globular proteins.
Hydrophobic side chains (e.g., Val, Leu, Ile, Phe) are buried inside the protein to avoid water.
Hydrophilic side chains remain on the surface, interacting with the aqueous environment.
Helps stability because
The exclusion of hydrophobic side chains from water acts like a "push," encouraging the protein to fold into a compact shape.
This folding reduces the system’s free energy, making the structure thermodynamically stable.
Creates a Stable Core
The protein core becomes tightly packed with hydrophobic residues, creating van der Waals interactions and dense packing.
This core acts as a scaffold that supports the overall folded shape.
how do disulfide bonds help provide stability
Disulfide Bonds (Cys–S–S–Cys):
These are covalent bonds formed between two cysteine residues.
They provide extra stability by crosslinking parts of the polypeptide chain.
These can link:
Different parts of the same polypeptide
Or even two separate chains
how do Electrostatic (Ionic) Interactions help?
Also called Salt Bridges
Occur between R-groups with opposite charges, e.g.:
Lysine (positive) and Glutamate (negative)
These form ionic bonds that stabilize the folded protein.
💡 Strongest when buried inside the protein where water is excluded (since water would shield charges and weaken the bond).
how does Hydrogen Bonds (H-Bonds) Between R-Groups help?
These are additional H-bonds beyond those in α-helices and β-sheets.
Occur between polar side chains, like:
–OH (e.g., serine, threonine)
–NH or –COOH groups
Contribute to fine-tuning the 3D shape and stabilizing specific local conformations.
what is Metal Chelation (Metal Ion Binding)?
Metal chelation is a process where a metal ion binds to a molecule (ligand) through multiple coordination sites, forming a stable ring-like complex.
Some proteins are stabilized by divalent metal ions like:
Mg²⁺ (magnesium)
Ca²⁺ (calcium)
Zn²⁺ (zinc)
These metal ions form coordinate bonds with electron-donating atoms in amino acid side chains.
Common residues involved: in chelation binding?
Histidine (His) → via its nitrogen in the imidazole ring
Aspartate (Asp) and Glutamate (Glu) → via carboxylate oxygen
carboxylate oxygen refers to the negatively charged oxygen atoms in a –COO⁻ group
how does metal chelation help with stabilization?(3)
Bridges Distant Regions
A metal ion can coordinate multiple residues that are far apart in the amino acid sequence.
This brings those parts of the protein closer together, helping the protein fold and stay folded.
Provides Rigidity
The metal-ligand bonds are strong, acting like molecular anchors to lock parts of the protein in place.
This is especially useful in enzymes or DNA-binding proteins where structure must be precise.
Stabilizes Active Sites
Metal chelation stabilizes active sites by firmly anchoring essential metal ions in the correct position, helping maintain active site structure and enhancing catalytic function.
Summary of Tertiary Structure Features:
Compact, Globular Shape
Thanks to the hydrophobic effect, disulfide bonds, ionic interactions, hydrogen bonds, and metal chelation, proteins fold into tight, stable 3D shapes.
These structures have minimal internal cavities and exclude water from their hydrophobic core.
Examples to Come
You’ll likely see specific protein models in class that demonstrate this globular, compact folding—like myoglobin or enzymes.
Thermodynamic Optimization
Tertiary folding is driven by minimizing unfavorable interactions (e.g., hydrophobic side chains exposed to water) and maximizing favorable ones (e.g., H-bonds, salt bridges).
The final folded form is typically the lowest-energy, most stable conformation.
key features of quaternary structure?
Stabilized by the same forces as tertiary structure:
Hydrophobic interactions
Ionic (electrostatic) interactions
Hydrogen bonds
Disulfide bonds
Metal ion coordination
Shape and complementarity between subunits are critical for function.
what are polypeptides in multi chain protein called?
usually called subunits
what is an example of a protein with multiple subunits?
Example: Hemoglobin
Hemoglobin has 4 subunits:
2 alpha (α) and 2 beta (β)
These subunits interact through:
Shape complementarity
Hydrophobic surface matching
Ionic bonds
Hydrogen bonds
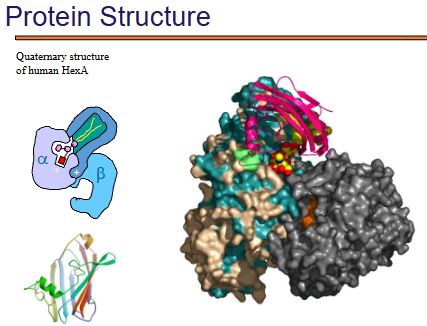
what is Quaternary Structure of Human HexA (Hexosaminidase A)?
This is an actual multi-subunit protein, with both α and β subunits.
The large molecular surface representation shows:
How tightly packed the subunits are.
How secondary structures (β-sheets, α-helices) are distributed across different subunits.
This illustrates the diversity and complexity of quaternary protein assemblies beyond simple dimers or tetramers (like hemoglobin).
what is the Folding Pathway for proteins?
Proteins must fold from a linear chain → native 3D shape.
Misfolding can lead to aggregates—clumped, non-functional proteins (often toxic, e.g. in Alzheimer’s).
Folding may involve intermediates, which are unstable and prone to misfolding.
intermediates are temporary, partially folded structures that proteins pass through as they fold into their native shape.
what is a chaperones?
A chaperone is a protein that helps other proteins fold correctly.
Prevent misfolding and aggregation (clumping) of newly synthesized or stress-denatured proteins.
Assist proteins in reaching their native, functional 3D structure, Help stabilize partially folded intermediates.
Some chaperones even help refold misfolded proteins or target them for degradation if they can’t be salvaged.
what is hsp70?
Hsp70 (Heat shock protein 70) is a molecular chaperone that assists folding:
Binds to unfolded or partially folded proteins.
Uses ATP to switch between:
Low-affinity (open) form
High-affinity (closed) form (with Hsp40 co-chaperone)
Helps prevent aggregation and promotes correct folding into the native state.
what guides protein folding? (3)
Folding is guided by the amino acid (AA) sequence — this is called Anfinsen’s principle.
Folding follows a unique, stepwise path that varies by protein.
Even with modern computing, predicting folding for anything beyond small peptides is very challenging.
what is Protein Denaturation and what is it caused by?
Denaturation = unfolding of a protein (loss of native structure).
Often caused by:
Heat
pH extremes
Organic solvents or detergents
Urea or guanidinium chloride
key characteristics of protein denaturation (3)
The energy difference between folded and unfolded states is small (~5–10 kcal/mol), so many proteins are easily denatured.
Only a few proteins can refold spontaneously (like ribonuclease); most require chaperones to regain proper structure.
Denatured proteins tend to aggregate, forming non-functional clumps — e.g., egg white proteins when cooked.
what is a Real-World Example of protein denaturation?
When egg white is cooked, the proteins denature:
Heat exposes hydrophobic R-groups to water.
This leads to aggregation and insolubility (why the clear egg white becomes white and solid).
what is the most common cause of denaturation?
Heat (>55°C for most proteins) — breaks weak bonds
why does ph changes denature proteins?
pH extremes — disrupt hydrogen and ionic bonds
high concentration of what kind solute denatures proteins?
High concentrations of certain solutes which disrupt the H-
bonding system of water (8M urea, 6M guanidine
hydrochloride) are excellent protein denaturants, and keep
the denatured form in solution.
Importance and Variety of Proteins
Proteins are the most abundant molecules in cells after water, making up 10–20% of cellular weight.
Humans make at least 25,000 different proteins, each with specific functions.
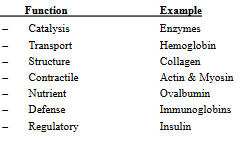
Types of Proteins: (2)
Some proteins are just simple polypeptides.
Others are conjugated proteins— are proteins that are chemically linked to a non-protein component, called a prosthetic group.
what is a Cofactors
non-protein chemical components required for activity.
Inorganic: metal ions (e.g., Fe²⁺, Zn²⁺)
Organic: vitamins or vitamin-derived molecules (e.g., heme, flavin, sugars)
what is a Prosthetic group?
a cofactor that is tightly bound (often covalently).
what is a Coenzymes?
organic cofactors used by enzymes (e.g., NAD⁺, FAD, CoA)
how can cofactors be binded?
Covalently attached (prosthetic group)
Or non-covalently bound (looser association)
Looser association = is a non-covalent, weaker, and often temporary interaction between molecules.