week 9 and week 10a: gene therapy
1/30
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
31 Terms
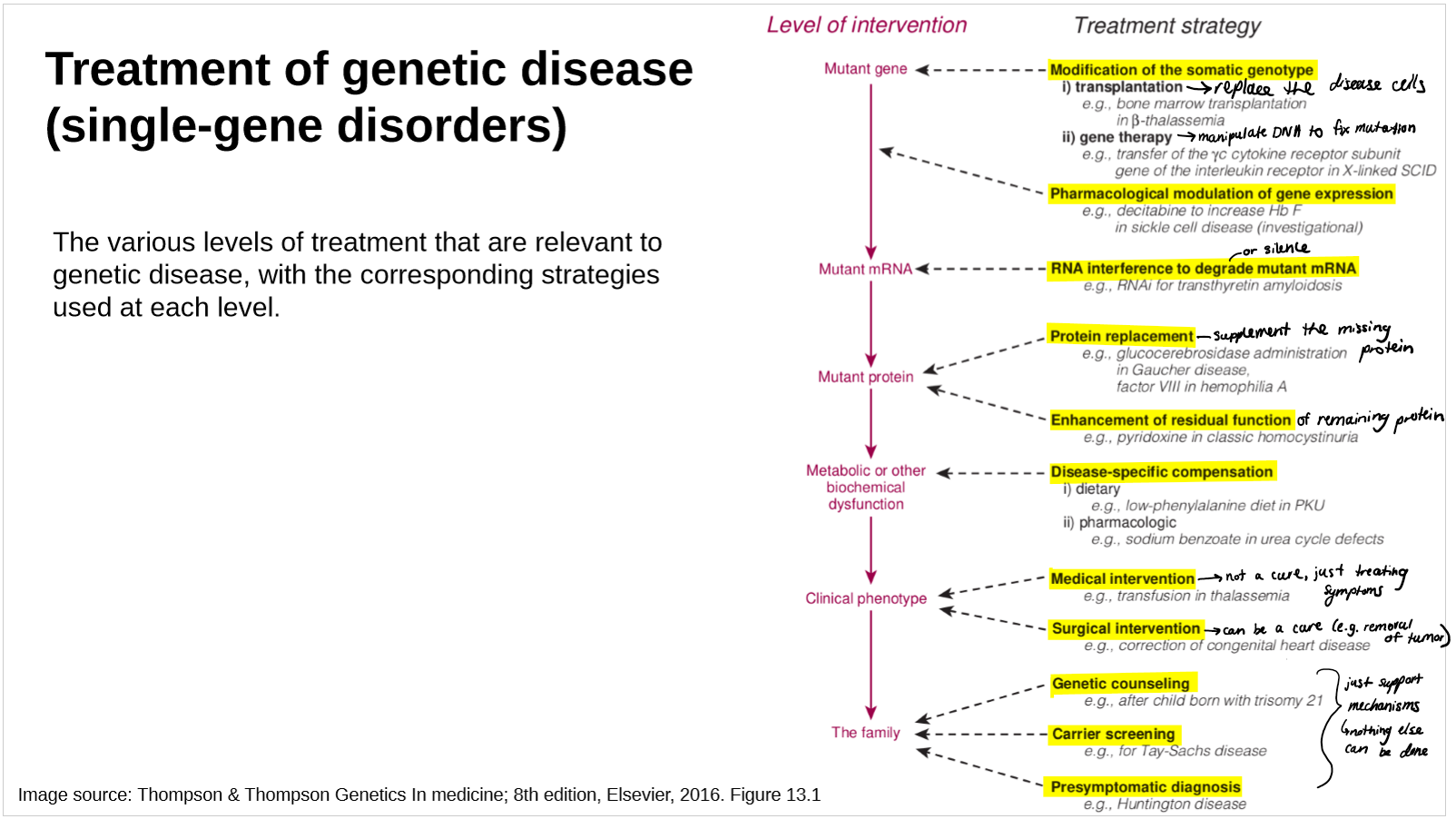
describe the different levels of gene therapy
what is gene therapy
manipulation of genes to cure a disease; introduction of therapeutic gene into
patient’s cells
● adding DNA to the genome
● altering the DNA of a gene
therapeutic gene/DNA
a gene/DNA whose expression will fight disease
biologics
Gene therapy products are a type of biologics (any pharmaceutical drug product manufactured in, extracted from, or semi-synthesized from biological sources; e.g. from blood, proteins, viruses, living organisms)
e.g. insulin
somatic cell gene therapy
DNA is transferred/edited in body tissues. Does not prevent the disease
from occurring in the next generation because it does not affect the germline. Many clinical trials and approved somatic cell gene therapy products
just fixes pts DNA, DNA is not passed down to next gen
germline gene therapy
genetic modification of germ cells. No country permits heritable human
genome editing in clinical practice; some countries allow use of genetically modified in vitro embryos in laboratory research. Criminal offence in Canada
gets past down to next gen
can eradicate genetic disease from population
unethical because can make designer humans
what does gene therapy require?
knowledge of target gene and cell/tissue, knowledge of gene function and disease
mechanism
need to know the type of mutation, what it is, where it is and what caused it
list and describe the two approaches of gene therapy
in vivo (inside the body): direct delivery into target organ (tissue easily accessible, cells hard to remove)
ex vivo (outside the body; cell therapy): removing cells from a patient, modifying them in a culture, reintroducing cells into the patient
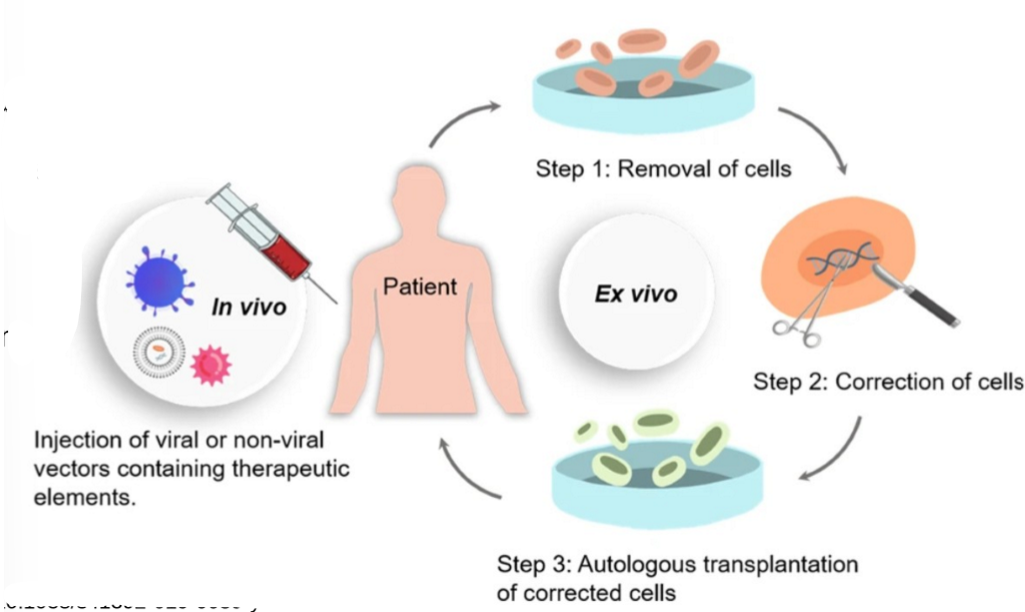
what are the basic steps of gene therapy
accurate diagnosis of the genetic defect/identification of DNA sequence
therapeutic DNA obtained using PCR, recombinant DNA tech, synthesis.
DNA is packaged into vector (gene delivery system)
vector is directly injected in target tissue or added to patient’s cells in culture
if ex vivo approach, cells are transplanted into patient
host’s machinery used to express new gene
what are the effects of gene therapy in terms of symptoms
May provide a longer-lasting effect than treating symptoms or supplying a protein/molecule.
Unclear if it provides a long-term cure
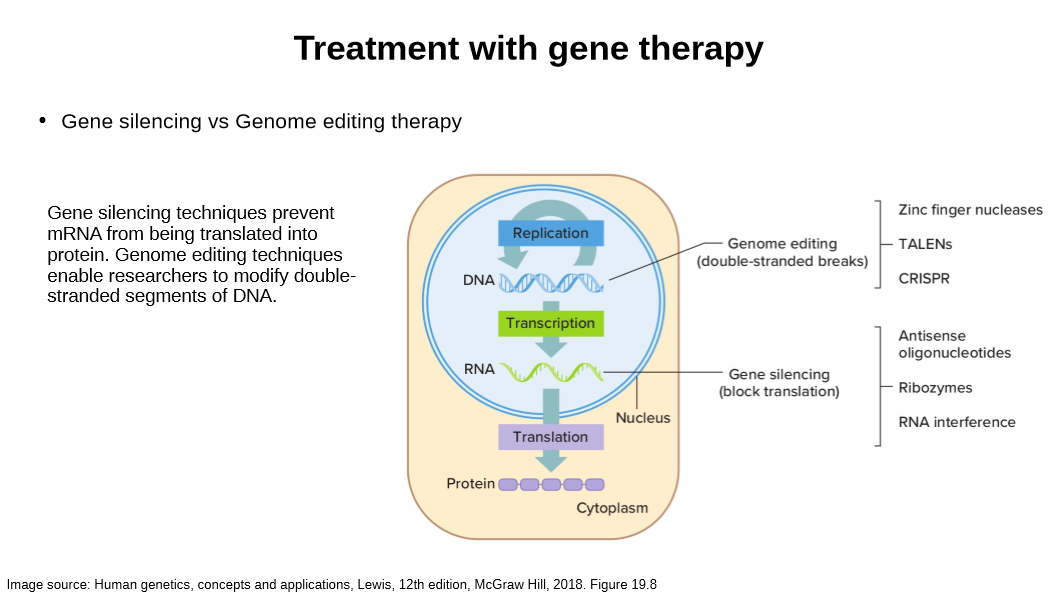
compare genome editing vs silencing
describe CRIPSR
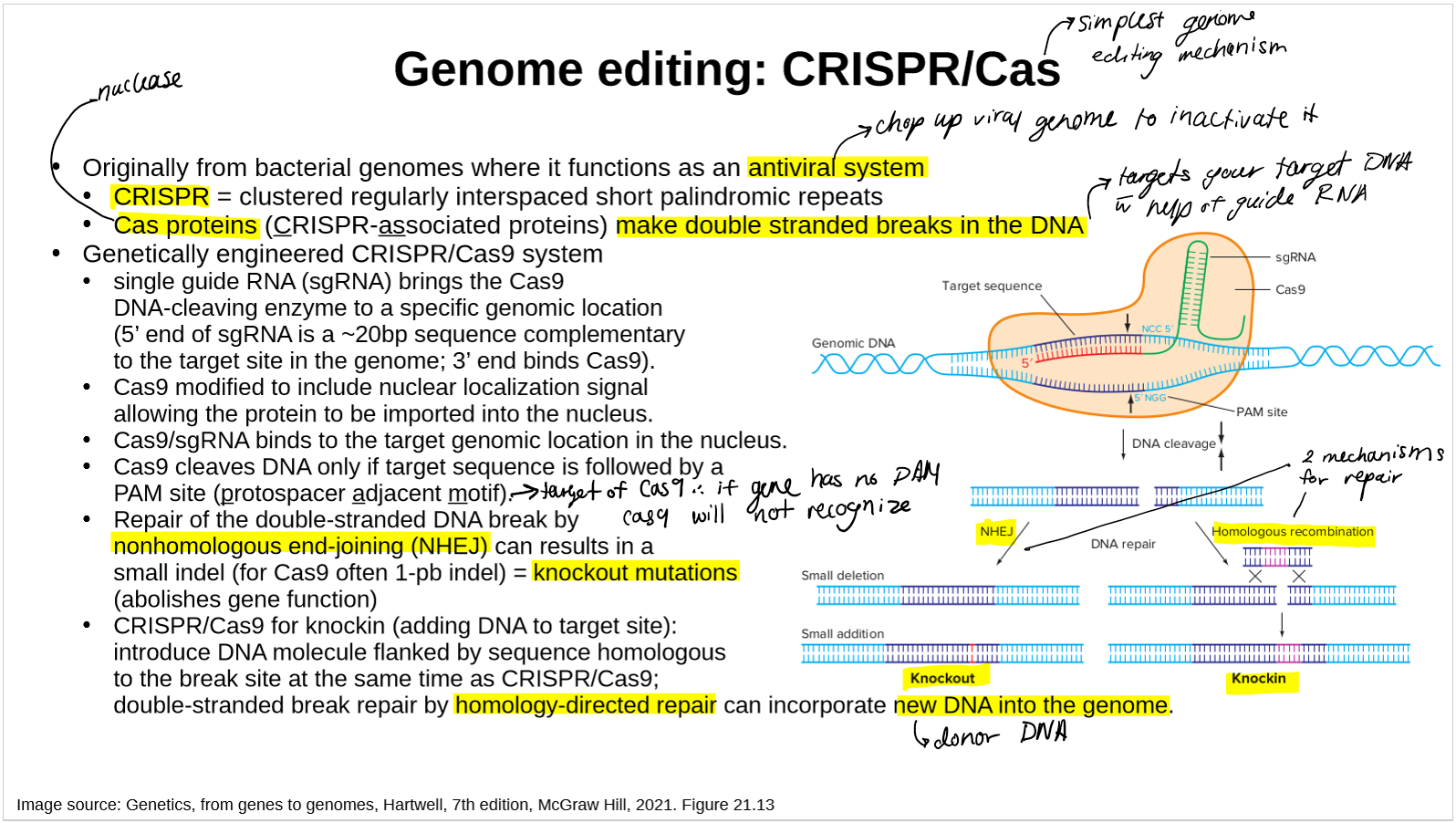
what is the major difference between ZFN/TALEN and CRISPR/Cas9?
ZFN and TALEN: protein-DNA system whereas CRISPR/Cas: RNA-DNA system
ZFN/TALEN: protein is specifically designed to recognize the target DNA and bring the nuclease to it
CRISPR/Cas9: RNA guide recognizes the target DNA and brings the nuclease
name the genome editing nucleases
what do they do?
Genome editing nucleases (ZFNs, TALENs and CRISPR/Cas9) induce DSBs at targeted sites. DSBs can be repaired by NHEJ or, in the presence of donor template, by HDR
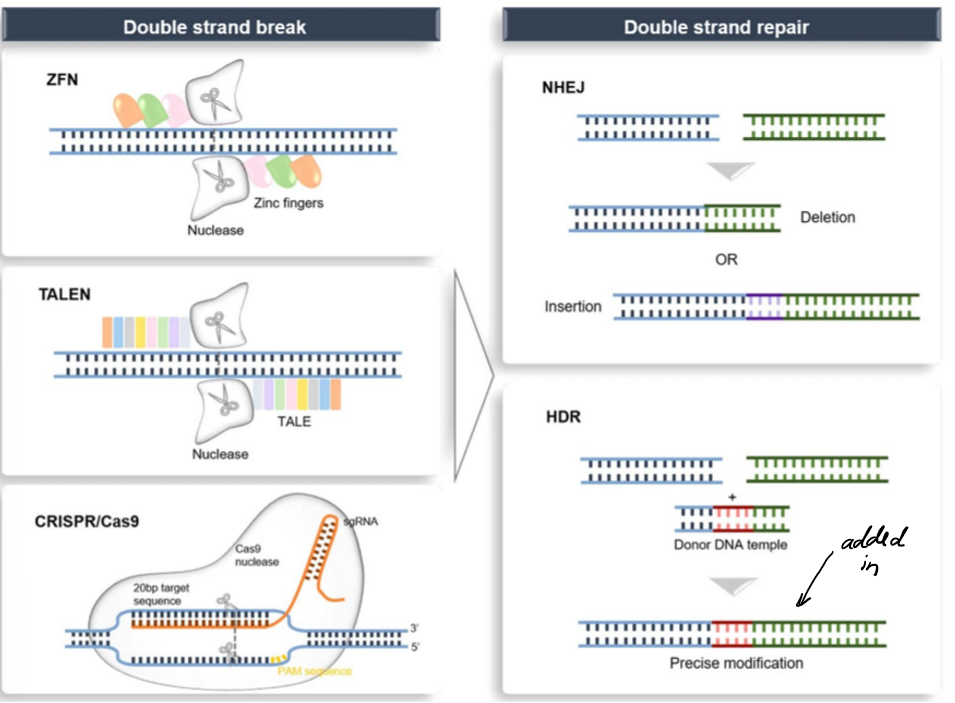
compare the gene editing methods for
size
nucleases
target sequence
specificity
limitations
difficulties engineering
difficulties delivering

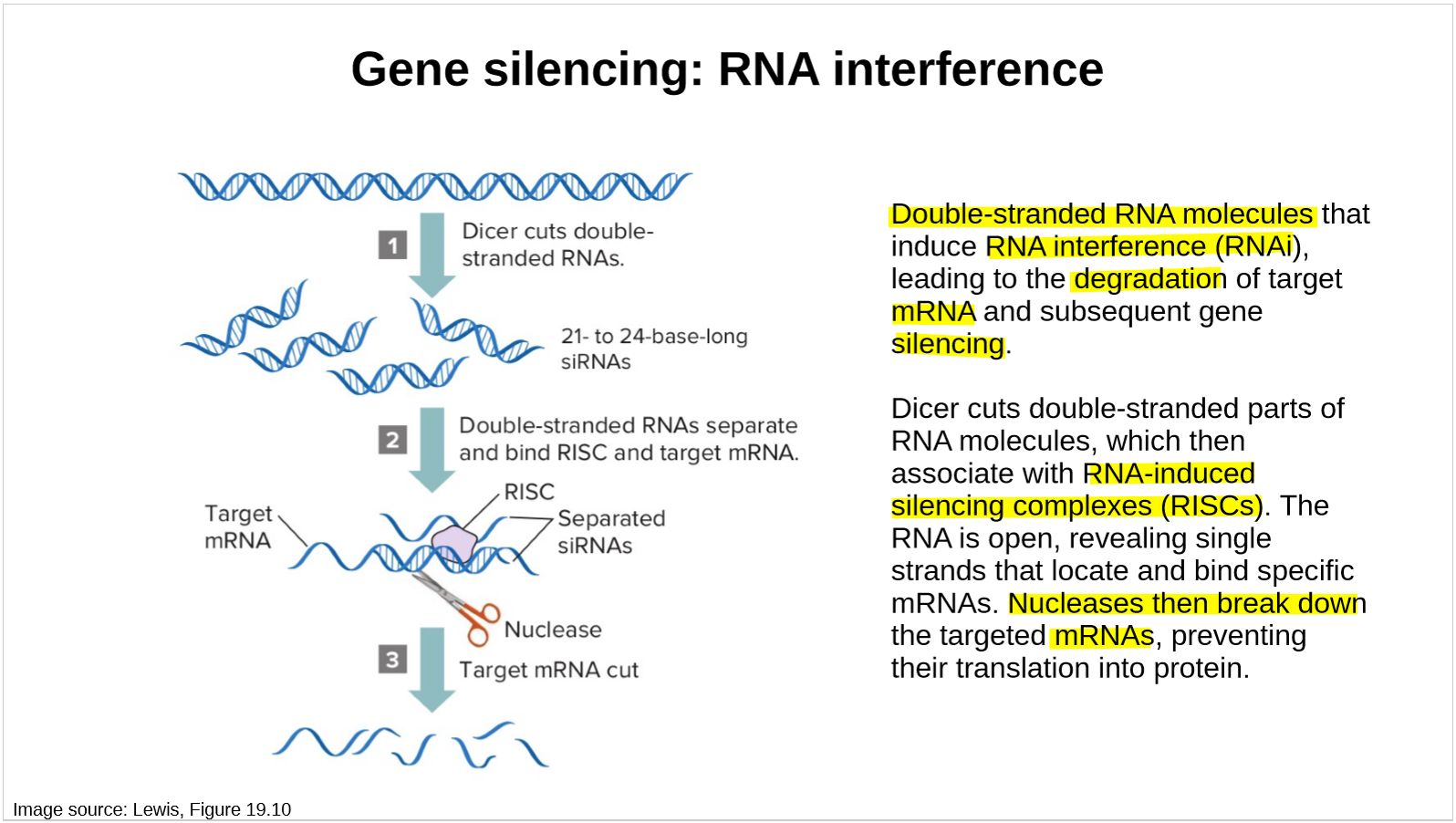
describe gene silencing by RNA interference
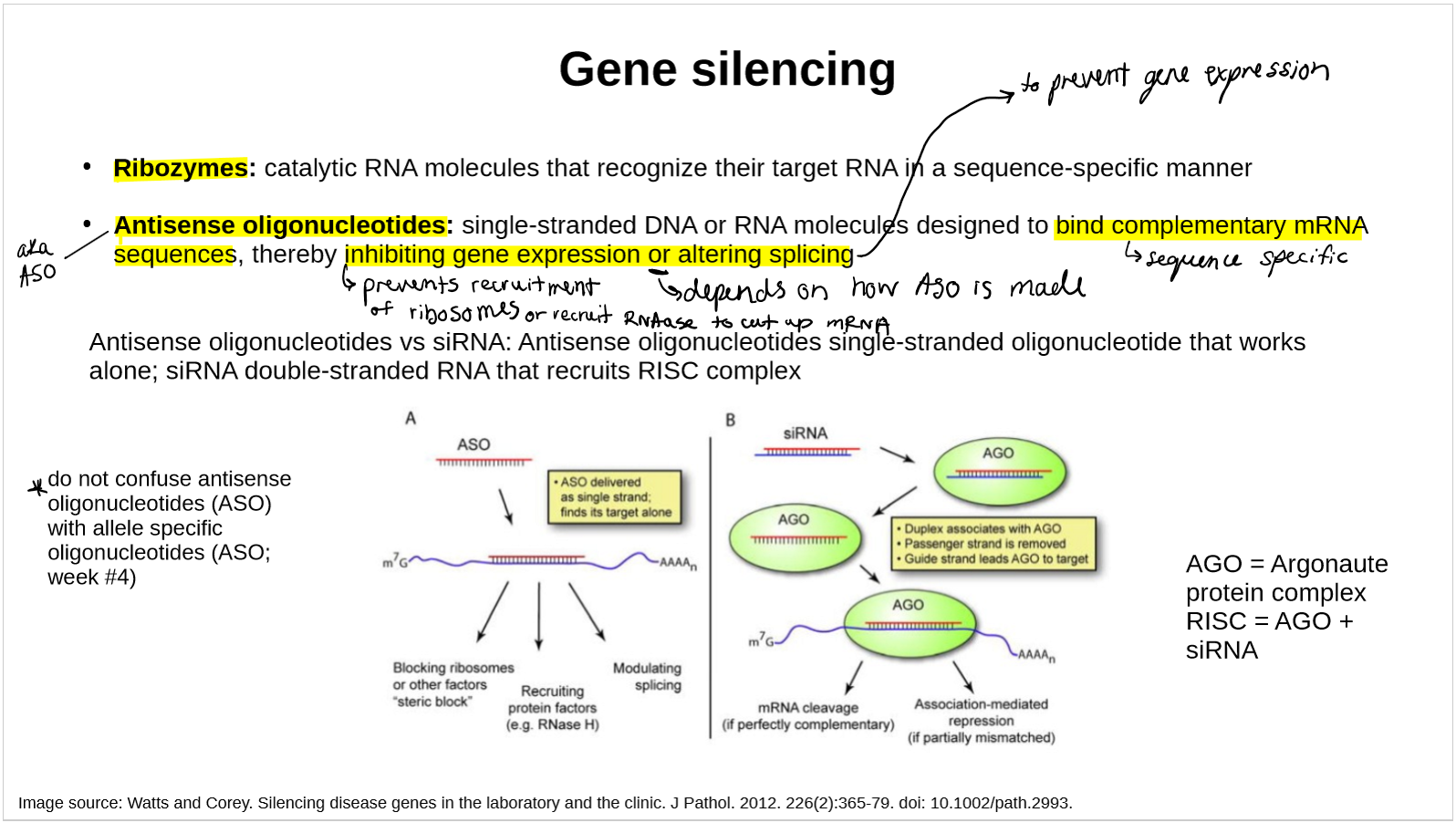
explain ribozymes’ gene silencing
catalytic RNA molecules that recognize their target RNA in a sequence specific manner
explain antisense oligonucleotides’ gene silencing
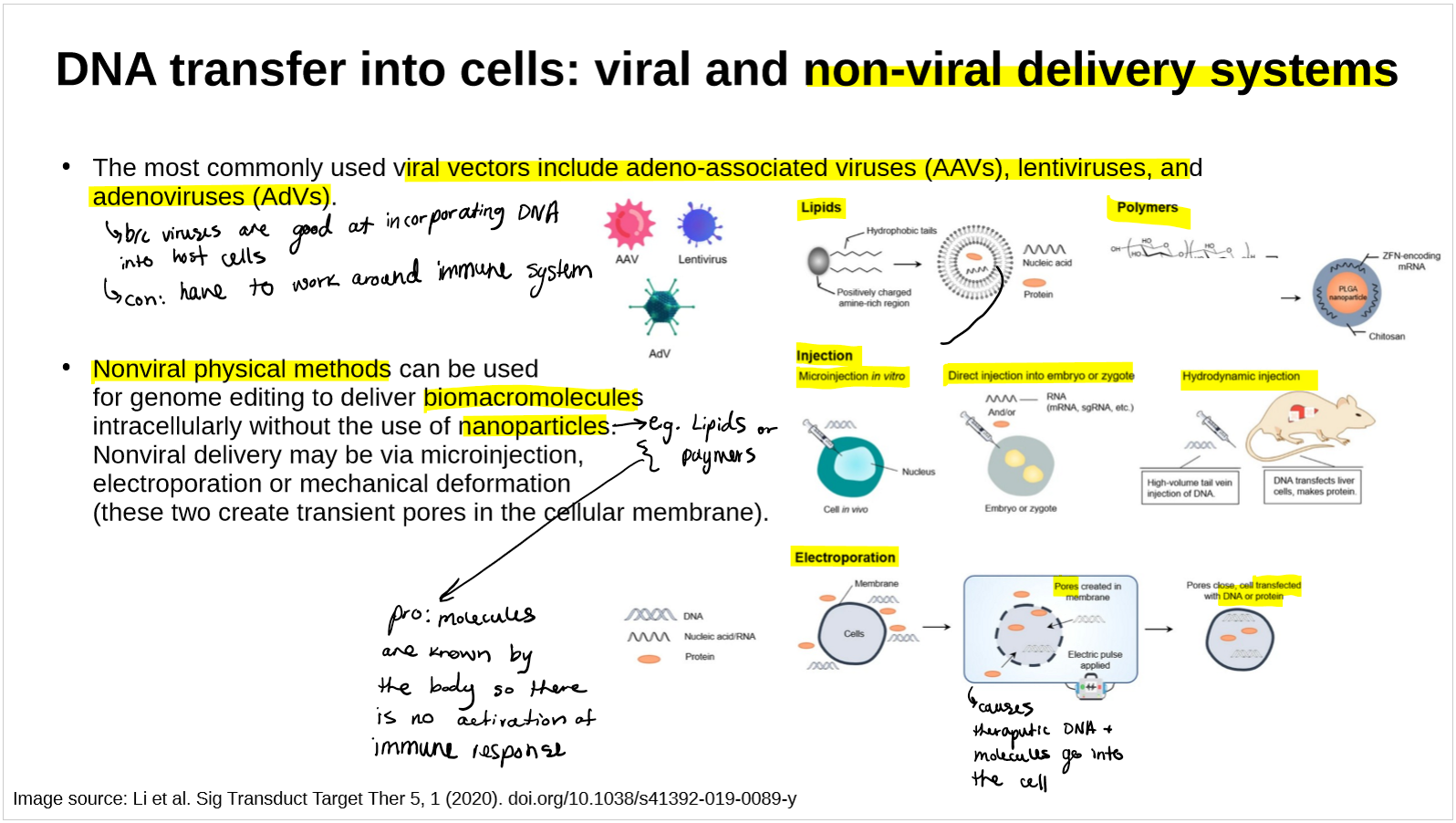
name and describe the types of nonviral physical methods of DNA transfer
how each works
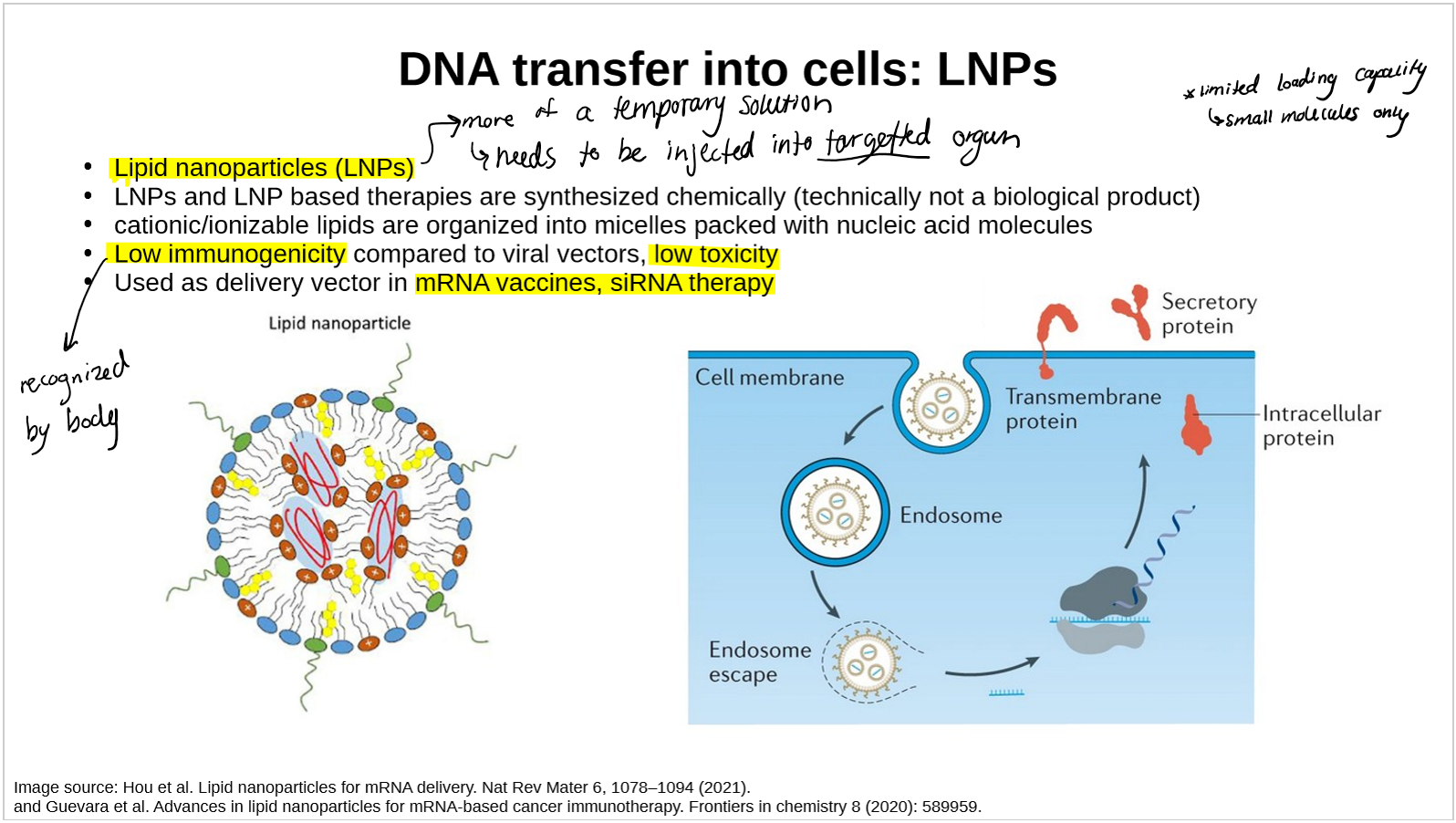
describe lipid nanoparticles (LPN) and how they are used to transfer DNA into cells
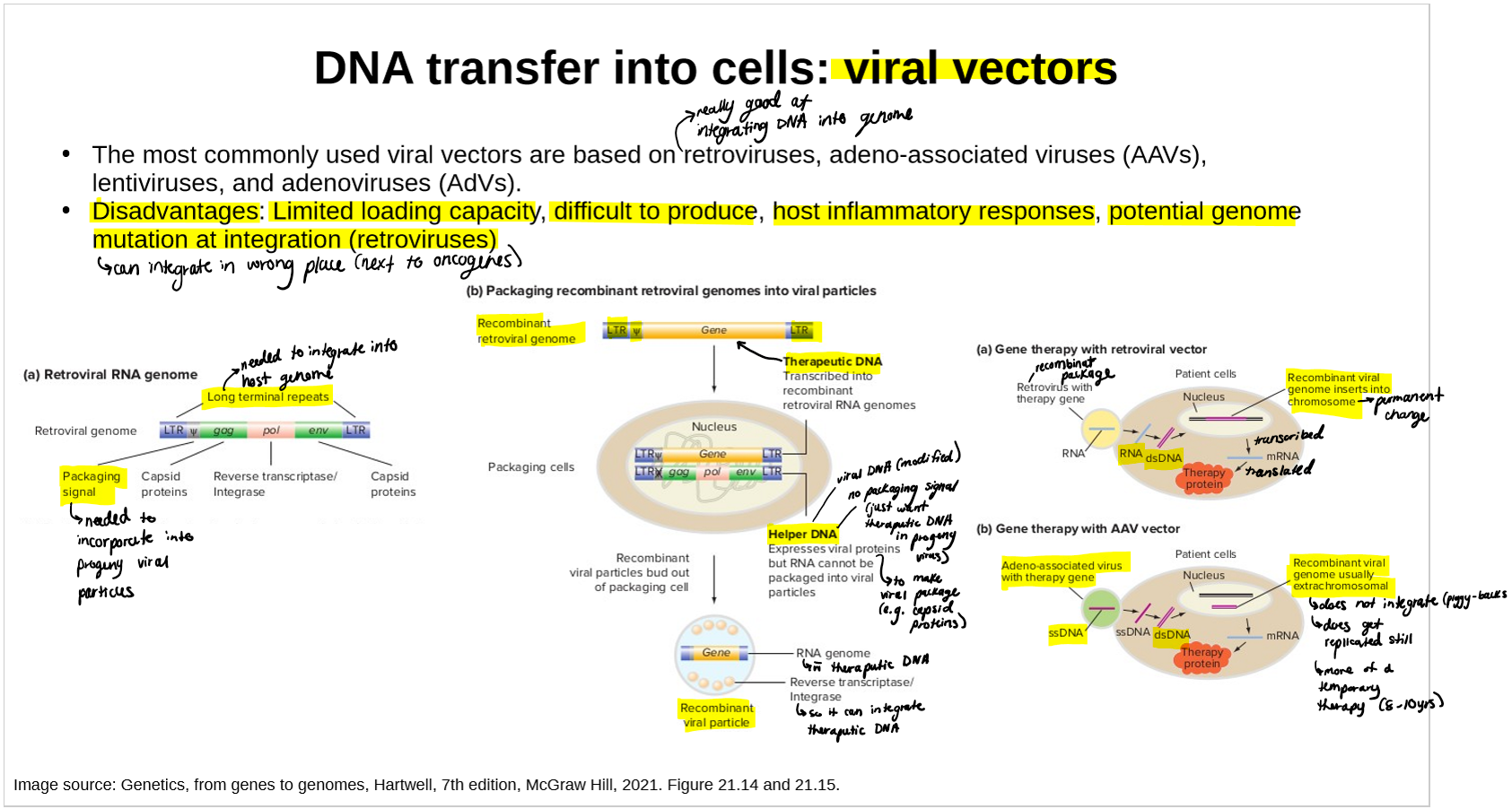
name and describe the types of viral methods/vectors of DNA transfer
how they work
limitations
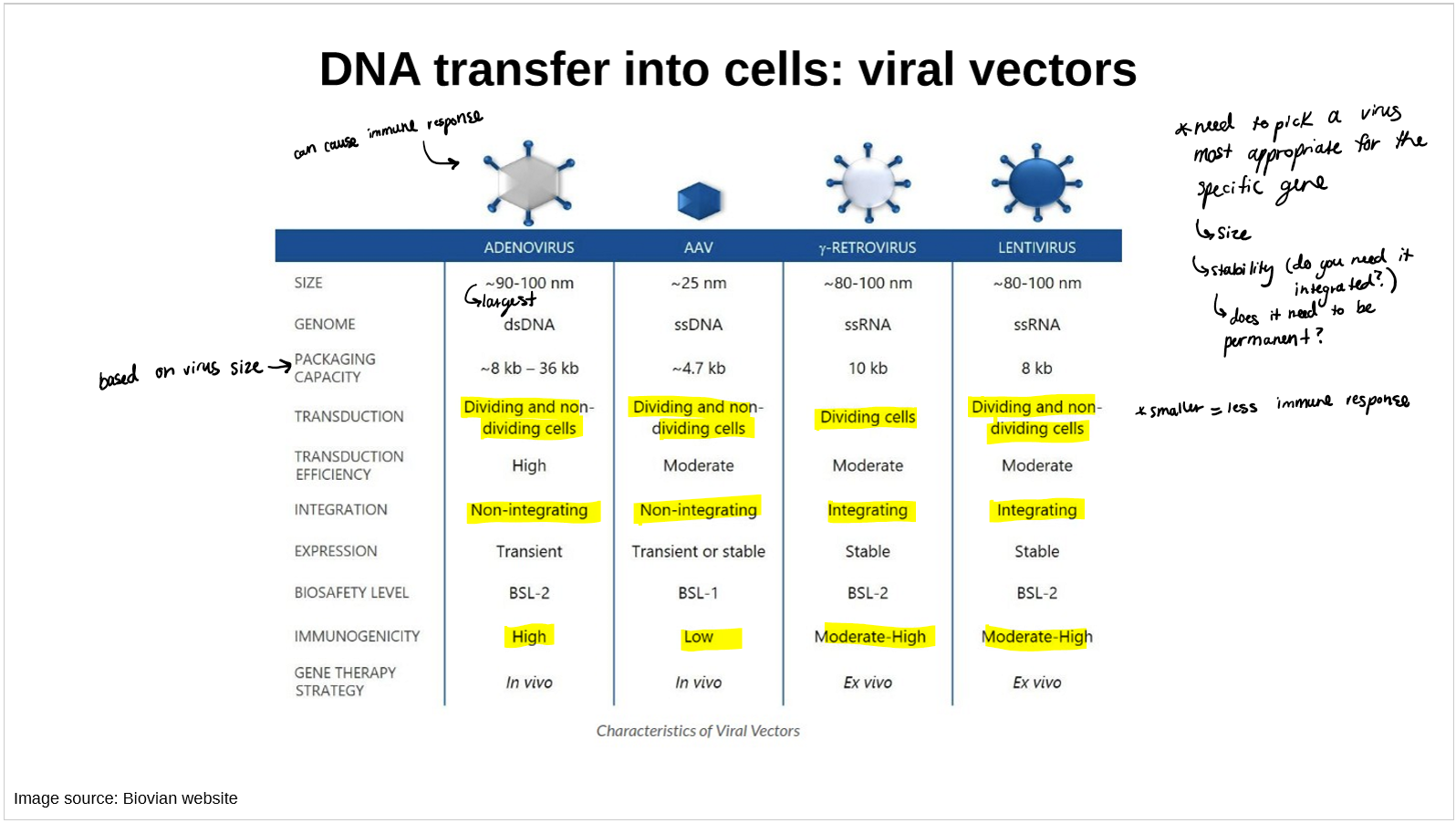
compare the 4 viral vectors for
size
genome
packaging capacity
transduction
integration
expression
immunogenicity
gene therapy strategy
compare allogenic and autologous gene therapy
allogenic: cells for gene therapy are from a matched donor then injected into pt
autologous: pts own cells are used for gene therapy
cells undergo gene therapy then are reintroduced to pt
no chance of the body rejecting the cells
what is Beta-thalassemia?
Autosomal recessive disorder; decreased production of hemoglobin
due to mutation in Beta hb chain
causes excess of alpha chains that aggregate and cause damage/anemia
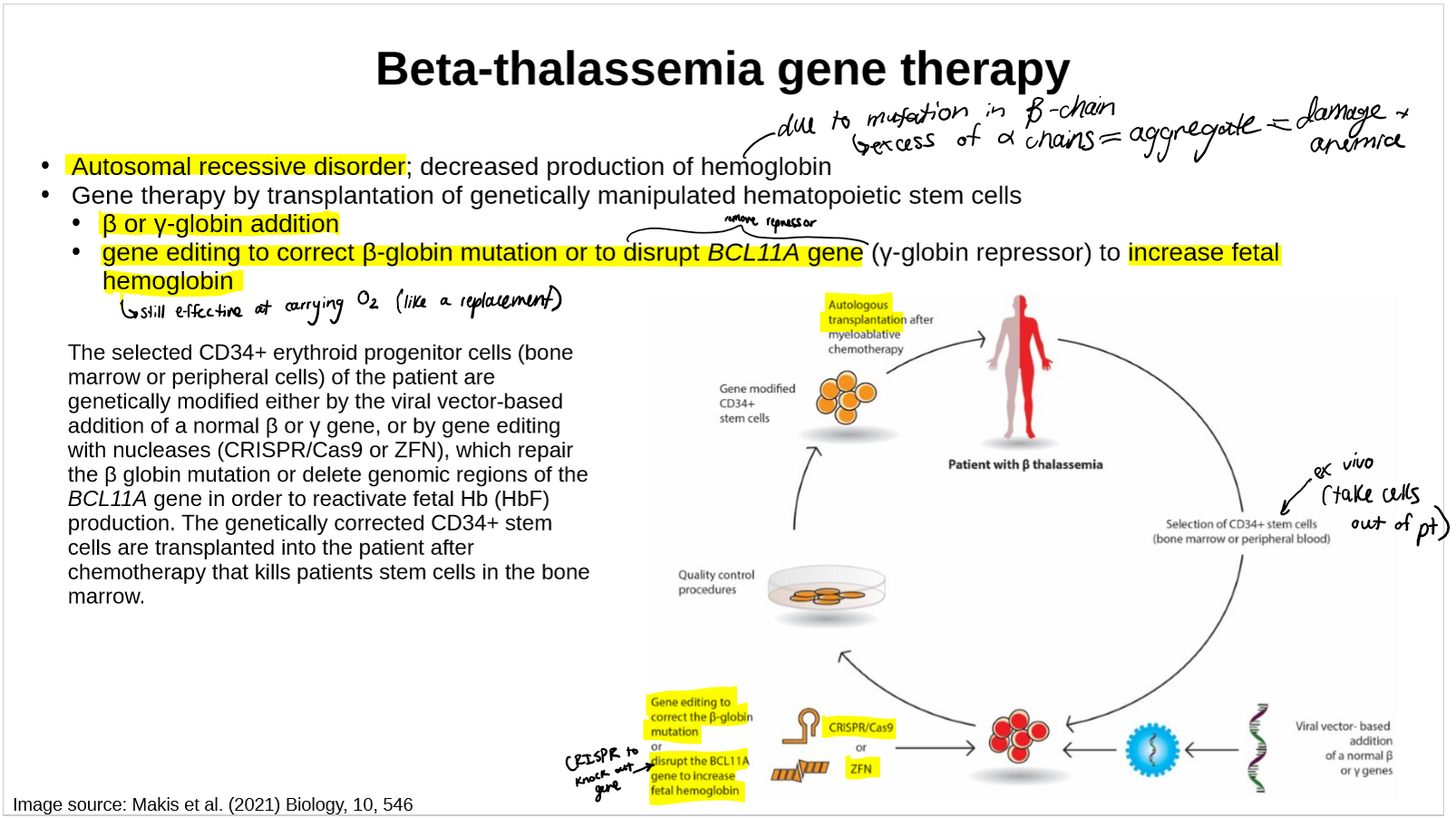
describe the gene therapy for Beta-thalassemia
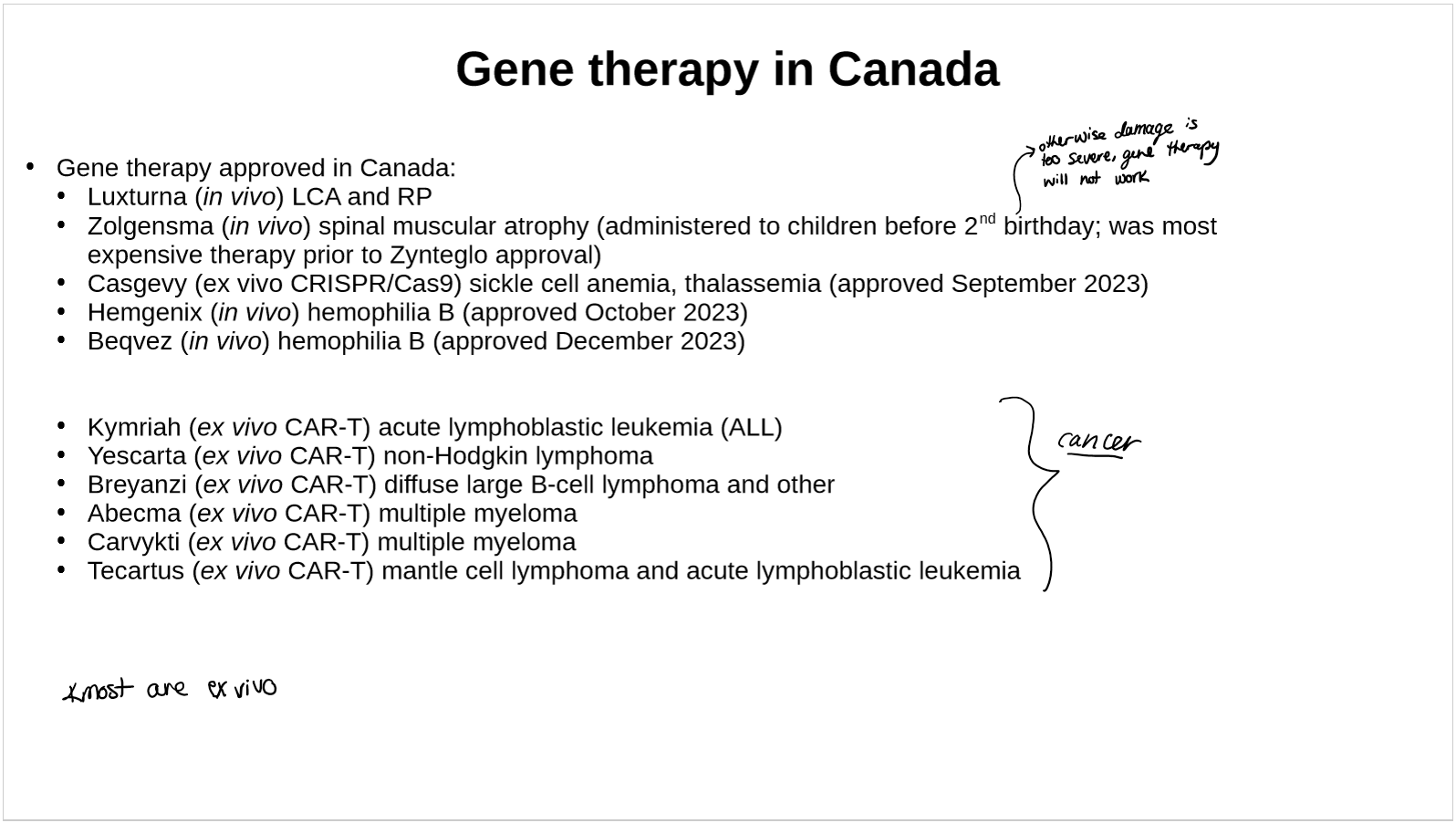
what is Casgevy
Casgevy (Vertex Pharmaceuticals Incorporated (Nasdaq: VRTX) and CRISPR Therapeutics)
For treatment of sickle cell disease and transfusion-dependent beta-thalassemia
first approved CRISPR gene editing treatment
can use the same treatment for sickle cell disease and transfusion-dependent beta-thalassemia because they have the same gene mutated
ex vivo CRISPR/Cas9 gene-edited therapy, in which a patient’s own hematopoietic stem cells are edited to produce high levels of fetal hemoglobin (HbF; hemoglobin F) in red blood cells.
Disrupts the BCL11A gene in red blood cell precursors in the bone marrow. Disruption of BCL11A reactivates the production of HbF (mimicing the HPFH phenotype).
get fetal Hb
what is Lyfgenia by BluebirdBio
At the same time also approval of gene therapy by BluebirdBio; Lyfgenia; $3.1 million; MOA: addition of Hb gene.
By September 2024: ~10 patients on Casgevy therapy, ~20 on Lyfgenia
More than 80% of U.S. patients are not suitable for the therapies, which are approved only for those over 12 with a history of severe pain crises. ("You have to be sick, but not too sick," Dr. Andrew Campbell, director of the Children's
National Comprehensive Sickle Cell Disease Program, Washington D.C)need chemo first, then transplant
cost is high
how many approved gene therapies are there globally? RNA therapies?
30 each
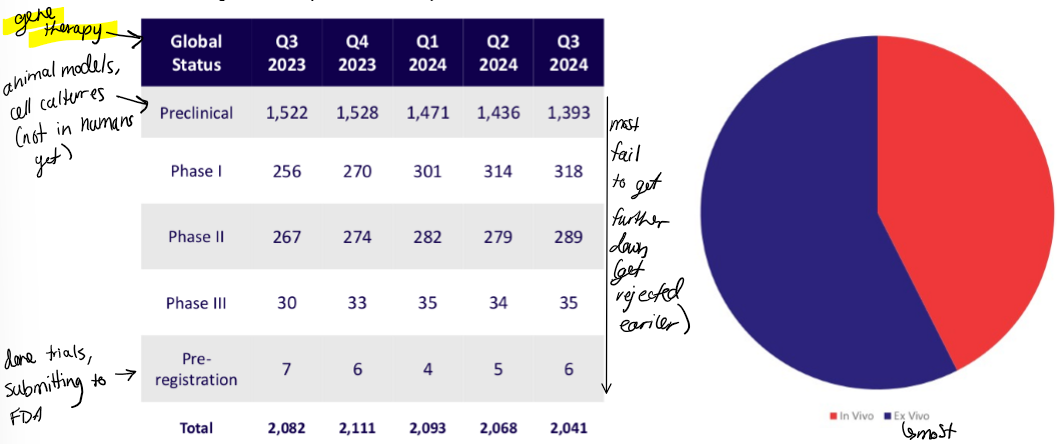
describe the current situation of gene therapy clinical trials
what stages are they in for the most part?
in vivo or ex vivo
most are ex vivo
most gene therapies are currently in preclinical phase (animal models)
very few get to pre-registration (submitting to FDA)
what are the majority of current gene therapy clinical trials and approved therapies trying to find a cure for?
cancer
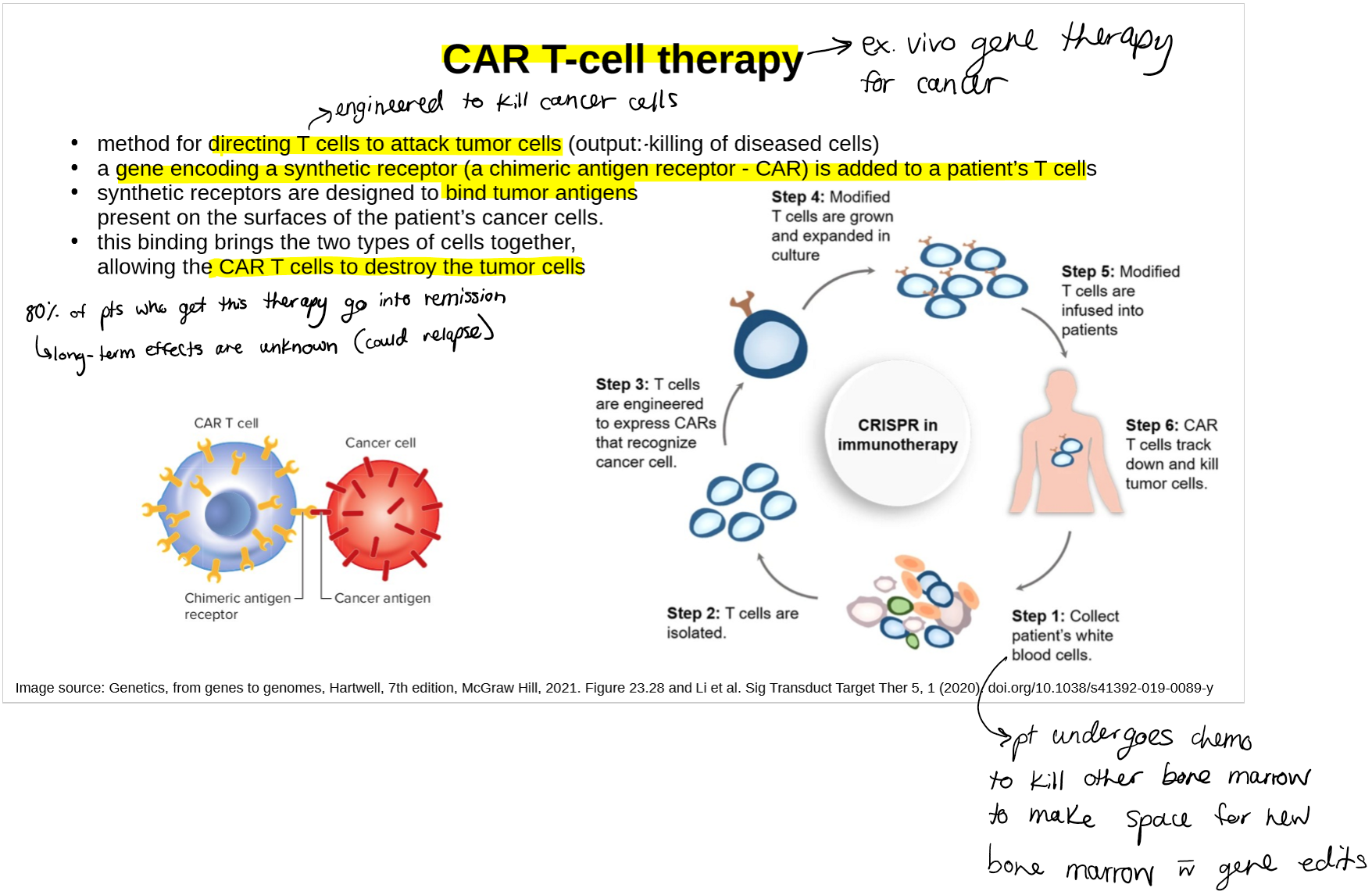
describe CAR T-cell therapy