WJEC AS Physics Unit 1.5 - Solids Under Stress
1/70
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No study sessions yet.
71 Terms
Hooke’s Law
The tension in a spring or wire is proportional to its extension from its natural length, provided the extension is not too great
Equation for Hooke’s Law
F=kx
Where F=Force (N), spring constant (Nm^-1) and x=displacement/extension (m)
How Hooke’s Law can be seen being obeyed on a Force-Extension graph
The line will be straight
Spring constant
The force per unit extension (Nm-1)
Area under a force-extension graph
The work done in deforming a solid
1/2Fx if Hooke’s law is obeyed
W=1/2Fx and F=kx may be combined to give W=1/2kx2
Graph of F against x
y-axis = F
x-axis = x
Gradient = k (spring constant)
Area under graph = Work done in stretching. 1/2Fx if Hooke’s Law obeyed
Tensile stress
σ
Stress is the force per unit cross-sectional area when equal opposing forces act on a body.
Unit; Pa or Nm-2
σ = F/A
When a force is applied to the surfaces of a solid material, it develops a stress
Equation for tensile stress
σ = F/A
Units of tensile stress
Nm-2 or Pa
Tensile strain
ε
Strain is defined as the extension per unit length due to an applied stress.
Unit: none
Dimensionless as = m/m. its base units are m * m-1 which = 1, thus having no units.
ε = ∆l/l
Equation for tensile strain
ε = ∆l/l or Extension (x)/Original Length (L)
Units for tensile strain
None. Dimensionless
Young Modulus
E
Young modulus, E = tensile stress/tensile strain or E = σ/ε when Hooke’s Law applies
Unless otherwise indicated this is defined for the Hooke’s law region.
Unit: Pa or Nm-2
Gradient of elastic region of stress-strain graph
Gradient of a stress-strain curve
Young Modulus, E
Work done in deforming a solid
Equal to the area under a force-extension graph, which is 1/2Fx if Hooke’s law is obeyed
W=1/2Fx and F=kx may be combined to give W=1/2kx2
Equations for energy stored in a spring
E = ½ Fx Where E = energy, F = force, x = distance and k = spring constant. |
E = ½ kx2 Where E = energy, F = force, x = distance and k = spring constant. |
Classification of solids
3 types of solid;
Crystalline
Amorphous
Polymeric
Classification of solids as crystalline
Solid consisting of a crystal, or of many crystals, usually arranged randomly. The latter is strictly a polycrystalline solid.
Metals are polycrystalline.
Regular, repeating pattern
Classification of solids as amorphous
A truly amorphous solid would have atoms arranged quite randomly. Examples are rare. In practice we
include solids such as glass or brick or ceramics
no long range order in the way atoms are arranged, though there may be ordered clusters of atoms.
Classification of solids as polymeric
A solid which is made up of chain-like molecules.
Formweed when many monome units are joined together
e.g.; rubber
Equation for Young Modulus
E=σ/ε
Units of Youngs Modulus
Dimenionless
When Young’s Modulus applies
When Hooke’s Law applies
Grains
large number of interlocking crystals
Orientation of crystal planes=random from one grain to the next
Grain boundaries have a larger component of impurity atoms which are forced out of the grains during crystallisation
Strain hardening
Molecules stretched in one direction and compressed in another
Grain boundaries
The boundaries between crystals (grains) in a polycrystalline material
Polycrystalline solid
Solid consisting of many crystals arranged randomly
E.g. metals
Ductile material
A metals which can be drawn out into a wire
This implies that plastic strain occurs under enough stress
Elastic strain
Strain that disappears when the stress is removed, that is the specimen returns to its original size and shape
Plastic strain
Strain that decreases only slightly when the stress is removed. In a metal, it arises from the movement of dislocations within the crystal structure
Effects of dislocations
A combination of grain boundaries and dislocations is responsible for the mechanical properties of polycrystalline metals
Metals can be strengthened by introducing barriers to dislocation movement, such as foreign atoms, other dislocations, and more grain boundaries
Strengthening of metals
Necking/Ductile Fracture
The characteristic fracture process in a ductile material
The fracture of a rod or wire is preceded by local thinning which increases the stress
Elastic limit
The point at which deformation ceases to be elastic
For a specimen, it is usually measured by the maximum force, and for a material, by the maximum stress, before the strain ceases to be elastic
Limit of proportionality
The point behind which Hooke’s law is not longer true. Beyond = elastic limit
Yield point
Just after the elastic limit
Point at which the material undergoes a large increase in strain for little/no increase in stress
Breaking point
material continues in the plastic region until it reaches its breaking point
Point on a stress-strain curve where the material fractures
Stress-strain/force-extension graph for a ductile material/metal
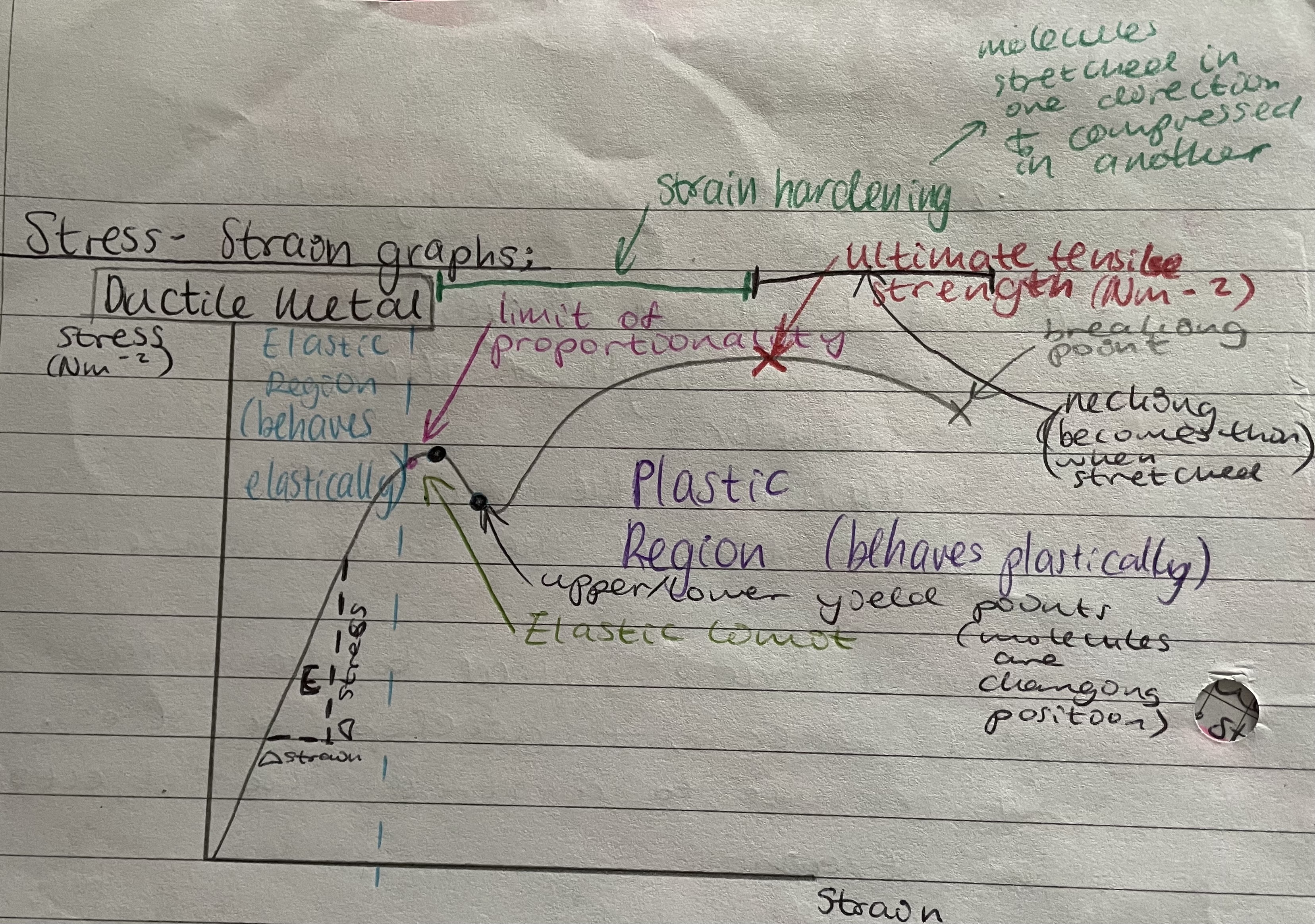
Elastic region of a stress-strain graph for ductile materials
The region where the material behaves elastically, gradient = young modulus and Hooke’s law is obeyed, undergoes elastic stretching
Linear portion of the graph before limit of proportionality is reached
Plastic region of a stress-strain graph for ductile materials
Strain hardening and then necking
Curve region of graph
Elastic limit of a stress-strain graph for ductile materials
Point on peak after limit of proportionality at which deformation ceases to be plastic, maximum stress before strain ceases to be elastic
Limit of proportionality of a stress-strain graph for ductile materials
Where liner region/elastic region comes to an end
Yield point of a stress-strain graph for ductile materials
Point in do just after EL
MOLECULES ARE CHANGING POSITION
Breaking point of a stress-strain graph for ductile materials
Point at end of graph at which the material fractures
Ultimate tensile strength
Nm^-2
The curve bend downwards behind the UTS
The maximum stress it can withstand while being stretched or pulled before it breaks
Ultimate tensile strength on a force-extension graph for ductile metal
Point at which curve begins to bend downwards
Process of elastic deformation/strain
For a material under low tension, separation between lattice particles is increased
Forces between the particles pull them back into their initial position when tension is removed
Process of plastic deformation/strain
Caused by an irreversible rearrangement of particles
Made possible by edge dislocations
Individual ions only move slightly
Ions next to and in edge dislocations drop into a lower PE position in the body plane
Extra ½ plane moves to right/direction force is acting in until it reaches the grain boundary
The crystal becomes elongated
The yield stress occurs at the yield point
The dislocation does not move back when the stress is removed so the elongation is plastic
What occurs after plastic deformation and factors which may affect this
edge dislocations; can get entangled, limiting their movement
Size of grains; larger, greater freedom of movement of the dislocations
Presence of point dislocations; foreign atoms can inhibit the movement of edge dislocations. A void in the lattice spawns more edge dislocations
Changing composition can affect this
Heating/quenching to make a metal more or less ductile
Cold working makes the metal more stiff and less ductile as causes dislocation entanglement
Irregulaities in the lattice of a ductile material
Dislocations
Edge dislocations
Point defect
In both types, combination of regular lattice, grain boundaries and dislocations is responsible for the mechanical properties of polycrystalline metals
Dislocations in crystals
certain faults in crystals which (if there are not too many) reduce the stress needed for plans of atoms to slide
Edge dislocation
The edge of an intrusive, incomplete ½ plane of atoms or ions present
Point defects
A lattice ion is missing or a foreign atom/additional ion is present
Brittle material
Material with no region of plastic flow, which, under tension, fails by brittle fracture
Stress-strain graph of a brittle materials
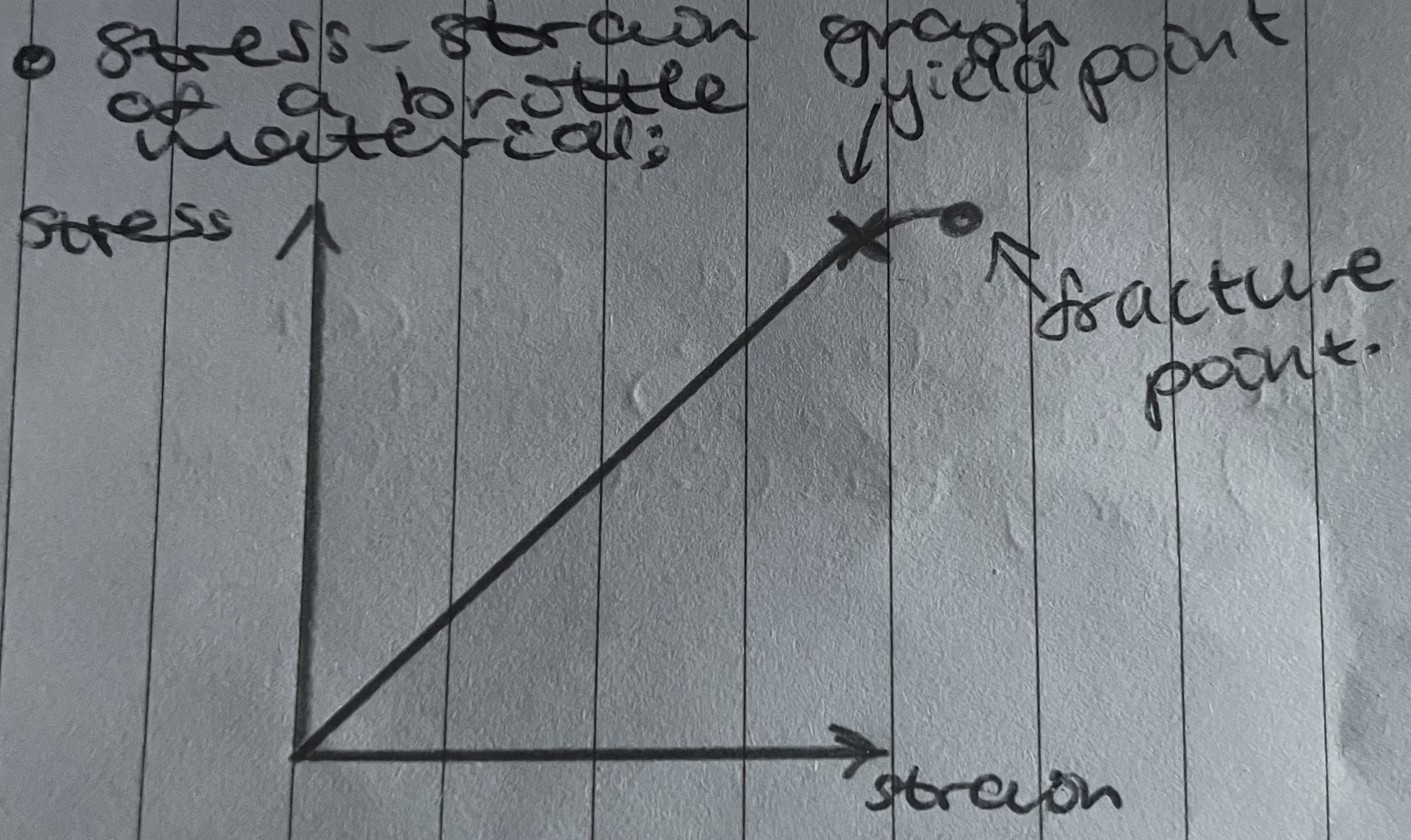
Elastic strain in a brittle material
Elastic strain occurs and Hooke’s law is obeyed up to fracture
Small elastic region before fracturing due to high Young Modulus
Brittle fracture
Fracture under tenting of brittle materials by means of crack propagation
Crack propagstion
Brittle materials undergo brittle fracture by crack propagation
Weakened by presence of minute cracks in its surface which break bonds
Stress at tip of cracks = concentrated and bonds break prematurely in the region, allowing the crack to move further through the specimen
Overloads the next bonds lower down at top of crack and process repeats itself rapidly until glass breaks
Effect of surface imperfections on breaking stress
Reduce breaking stress of a material as it will allow for cracks to develop in thin fibres
Effect of compression on breaking stress
Breaking stress can be increased by putting the surface under compression
A much larger stress is required to separate the two sides of the crack and to get the rest of the material into tension so it can separate
Force-extension graph for rubber
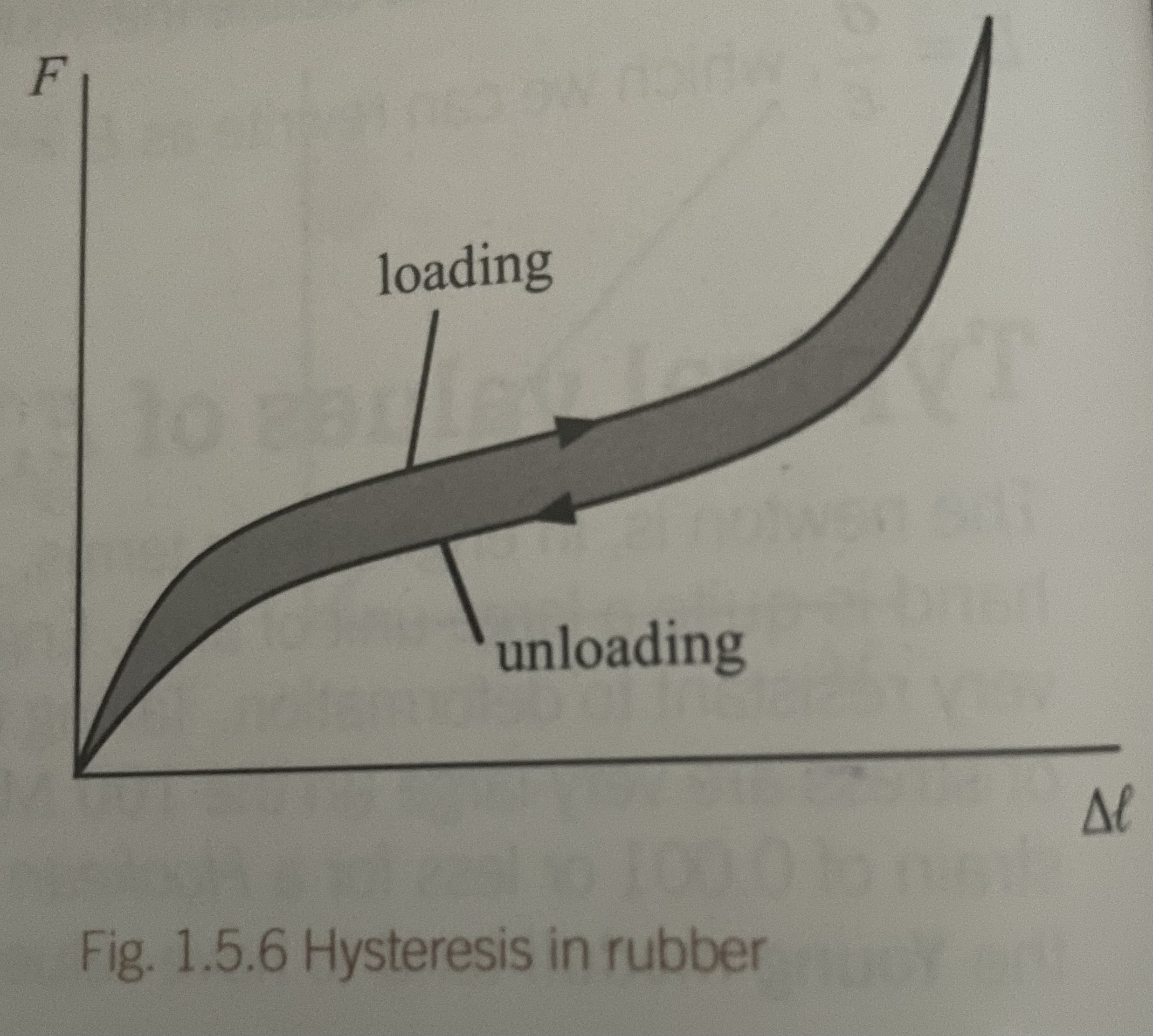
Chacreristsics of a stress-strain graph for rubber
Hooke’s law only approximately obeyed
Low Young modulus
Extension due to straightening of chain molecules against thermal opposition
Non-linear
Loading + unloading curves differ - elastic hysterisis
Exhibits large strain
Low stress
Volume stays roughly constant despite large extension
Area under a stress-strain graph for rubber
Energy lost as heat during 1 stretching/relaxing cycle. Shows elastic hysterisis effect
Young modulus of rubber
Non-linearity of graph means YM can either refer to;
gradient of tangent at origin
Value of stress/strain for a particular stress
Elastic hysterisis
When a material such as rubber is put under stress and the stress is then relaxed, the stress-strain graphs for increasing and decreasing stress do not coincide, but form a loop. This is hysterisis. Energy lost during stretching cycle for rubber
Permanent set
Load removed, permanent extension which may creep back to zero
Process of stress and strain on rubber
Start; Chain molecules are being uncoiled and no direct stress is applied to any bonds. High strain, low E, low increase in stress
End; long chain molecules have been straightened out and direct stress is being applied to the strong covalent C-C bonds along the backbone of the polymer. Very little strain achieved, line is steep and high E
Factors effecting the amount of extension
Original length of the wire
Diameter
Tension in the wire
The material the wire is made from
Rubber
can stretch to several time original L
Force required is much less than other types of materials, e.g. amorphous as bonds not stretched, just rotated
Presence of cross-linkages of entanglement between/of molecules limits total extension possible
The thermal motions of the atoms in the molecules, provide the opposition to the extension
T removed, random molecular motions of atoms re-randomised the shape of the molecules leads to contracting
Some energy is converted in KE by collisions. Therefore not as much work is done in contracting —> hysterisis effect
Examples of a ductile material
Metals such as copper
Examples of a brittle material
Glass/ceramics