Chapter 17 Innate Nonspecific Host Defenses
1/65
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
66 Terms
susceptibility
lack of resistance to a disease
Immunity
ability to ward off diseases
Innate immune system
defenses against any pathogen present since birth
Adaptive immunity
immunity or resistance to a specific pathogen that develops over time with exposure of pathogens
development of antibodies
Physical barriers that make up the first line of defense
skin (dermis and epidermis)
mucous membranes (mucus)
ciliary escalator
lacrimal apparatus (secretions help remove microbes)
skin
body’s largest organ that consists of
epidermis: made up of tightly packed cells that sloughs off to remove microbes
dermis: connective CT
Mucous membranes
susceptible to spirochete pathogens (treponema pallidumz)
add pic
Mucus
traps microbes that are produced by goblet cells
ciliary escalator
transports microbes trapped in mucus away from lungs using cilia
Lacrimal gland
produces tears that help wash away microbes from the surface of the eye
part of first line of defense
Tears and mucus
contain lysozymes that break apart bacterial cell wall
B. pertussis targets respiratory system and damages
cilia
B. pertusis uses virulence factors
filamentous hemagglutinin
fimbriae
pertacin
that allow it to bind to cilated cells
PTx is an
A-B exotoxin that stimulates adenylate cyclase which
reduces phagocytic activity
increases histamine release
PTx toxin stimulates adenylate cyclase which increases
cAMP in immune cells, weakening their ability to fight off infection
Histamine releaxe causes
vasodilation which makes blood vessels leaky leading to inflammation
controlled by PTx
B. pertussis (pathogen) controls the
fever → kills our cells
cytokines are
signaling molecules
Interleckins
TNF - tumor neurosis factor
Chemical barriers in first line of defense
sebum - inhibits bacterial growth
lysozyme - break down cell walls by destroying chemical bonds
gastric juice - has low pH that kills most pathogens
vaginal secretion - prevents yeast infections
resident microbiota
normal, non-harmful microbes that live in the human body
Vaginal canal normal microbiota produce
pH that is not suitable for Candida albicans
Commensal microbiota
microbes benefits without harming host
Probiotics with prebiotics for example LAB prevent
Salmonella enterica growth during antibiotic treatment
Fever is elevated body temperature triggered by
IL-1 from phagocytes, enhancing T-cell production, and interferon activity
Phagocytosis
involves neutrophils, macrophages and dendritic cells that engulf pathogens
Inflammation brings immune cells to infection sites via
vasodilation and permeability increases
Triggered by
Histamine
Kinins
Prostaglandins
Leukotrienes
Neutrophils
can leave blood, enter tissue, and destroy microbes
essential in early infection
Macrophages
clean up damaged tissues
increase in later infection
Natural killer (NK) cells
target infected host cells and tumor cells
Lymphatic system
drains interstitial fluid, filters lymph through nodes containing B-cells and T-cells
Nodes trap microbes for phagocytosis
Phagocytic cells
Neutrophils: lots of numbers
Macrophages: the marines
Dendritic cells:placed in epidermis
B-cells: create antibody
T-cells: activate B-cells
B-cells and T-cells are found in
lymph nodes + nodules
Cytotoxic T-cells (CTL’s) and NK’s target
virally infected cells and cancer cells
Eosinophils contain
granzymes + perforins that create pores in pathogen membranes
increase during parasitic infection
Mast cells/ basophils are
granulocytes that contain vasodilators
Vasodilators include
histamine
kinins
prostaglandin
leukotrienes
Macrophages start as
monocytes: immature macrophage
has no phagocytic activity
mature by leaving blood + entering into tissue
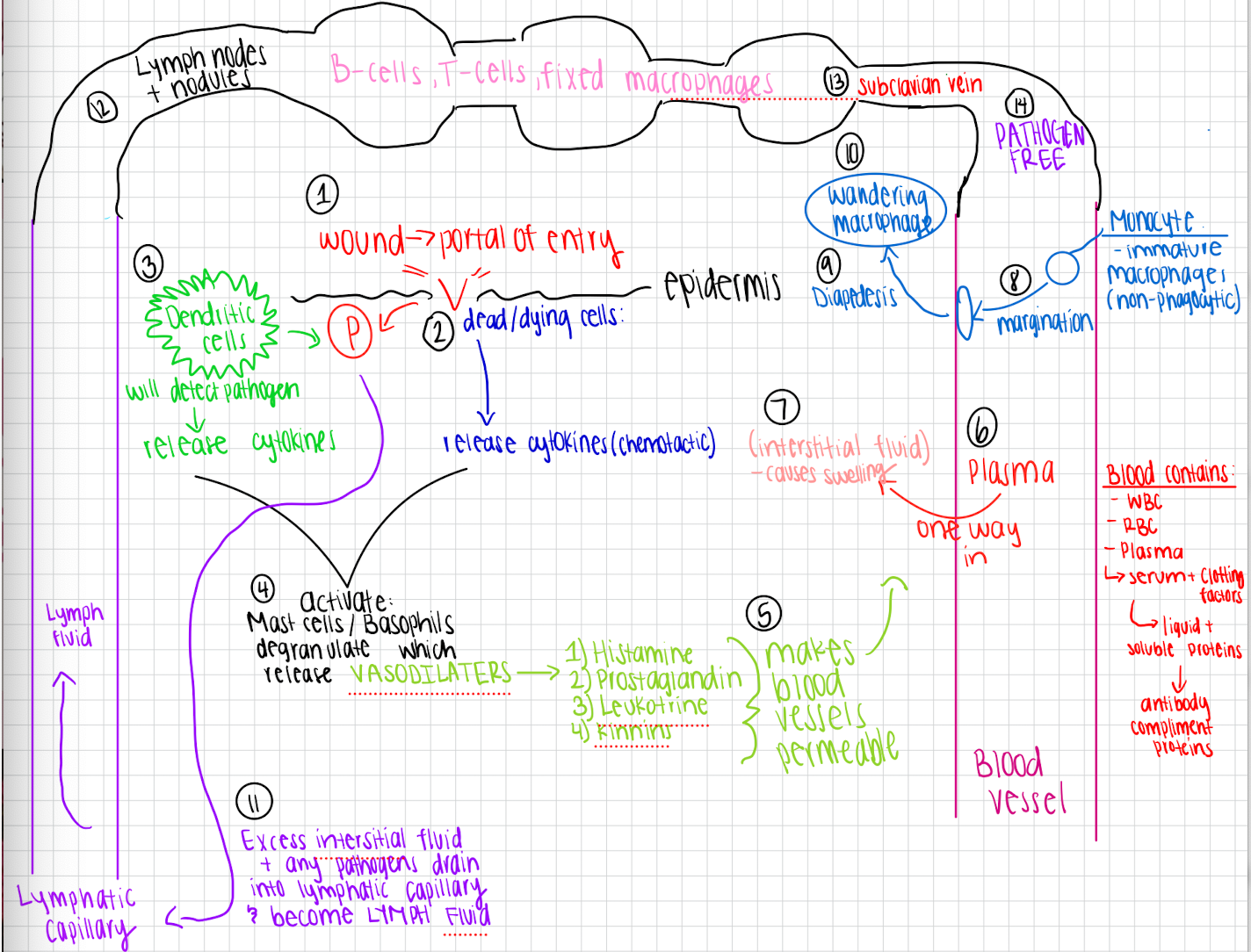
Inflammation molecular pathway
Wound creates a portal of entry in the epidermis
Dead/dying cells damaged by wound release chemotactic cytokines
Dendritic cells near wound detect that pathogens that are entering and release additional cytokines
Cytokines from both dying cells and dendritic cells activate mast cells and basophils
Mast cells and basophils degranulate which causes release of VASODILATORS
HISTAMINE
PROSTAGLANDIN
LEUKOTRINE
KINNINS
Vasodilators make blood vessels permeable allowing plasma to flow from blood vessel into tissue causing interstitial fluid
Monocytes move closer to vessel walls (marginatation)
Monocytes squeeze through blood vessel walls and enter the tissue (diapedesis) → wandering monocyte
Excess interstitial fluids along with any pathogen drains into lymphatic capillaries and becomes lymph fluid
Lymph fluid will travel through the lymph nodes and nodules that contain T-cells, B-cells, and FIXED macrophages
It then goes into subclavian vein where it is returned to the blood PATHOGEN free
Fluid exchanges between lymphatic system and blood
Blood plasma leaks into interstitial spaces, enters the lymphatic capillaries, and flows through lymph nodes. Lymph returns to blood through the subclavian vein
PAMP’s (Pathogen Associated Molecular Patterns)
Molecules on pathogens that trigger immune responses by being recognized as foreign
TLR’s (Toll-Like Receptors)
detect and bind to PAMP’s, initiating signaling pathways that activate immune response
Flagella
NAM-NAG
Mycolic acid
LPS
GP120
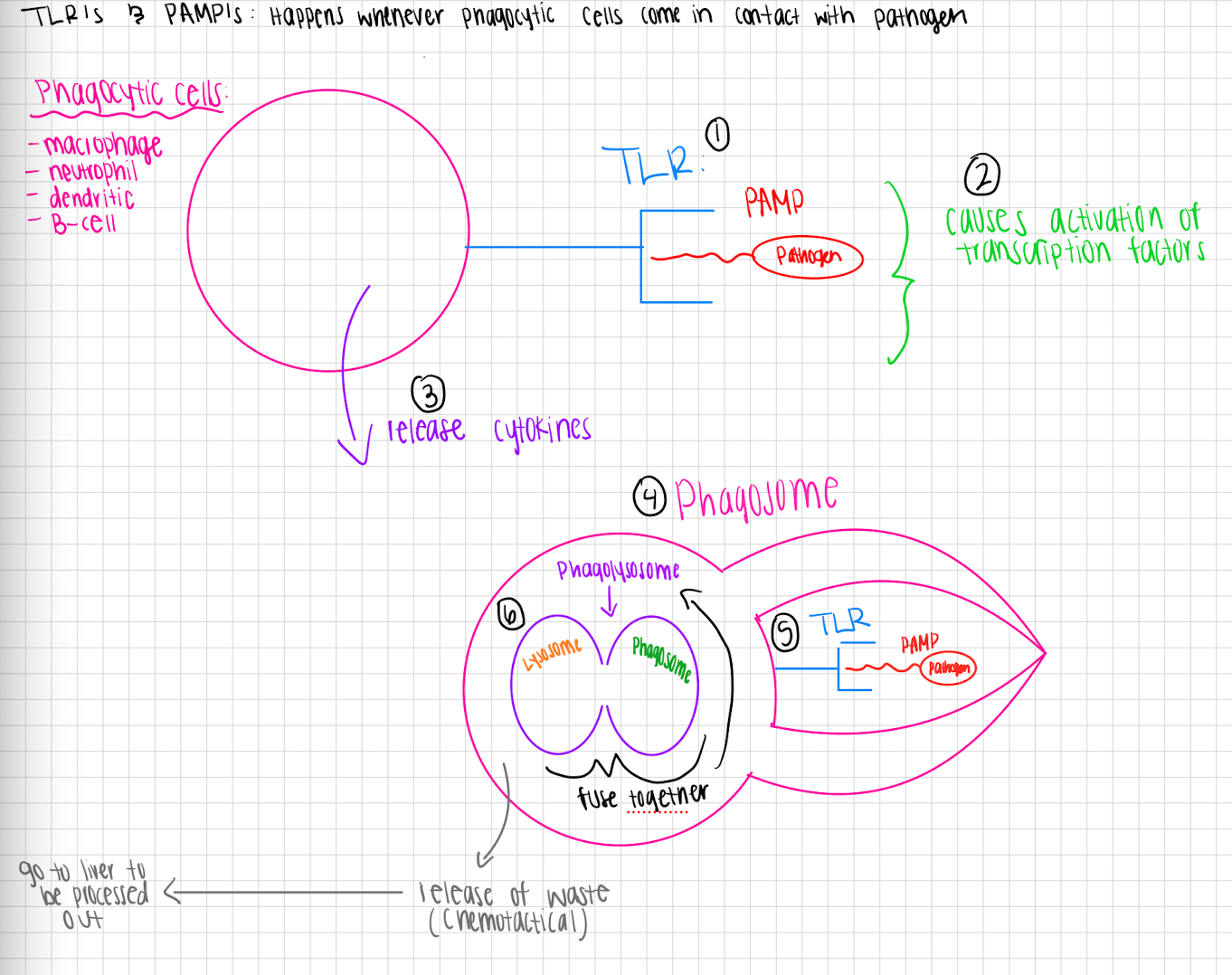
PAMP and TLR process (phagocytosis)
…
Phagocytosis process
Phagocytes recognize pathogens
Phagocyte surrounds pathogen with its membrane, forming a pocket around it
Phagosome gets created that contains pathogen
Phagosome fuses with lysosome to form phagolyssome
Enzymes in phagolysosome break down pathogen into smaller pieces
Excoystosis
Bacteria in a biofilm community are more difficult to
phagocytose than free floating bacterial cells because they are more difficult to be pulled off
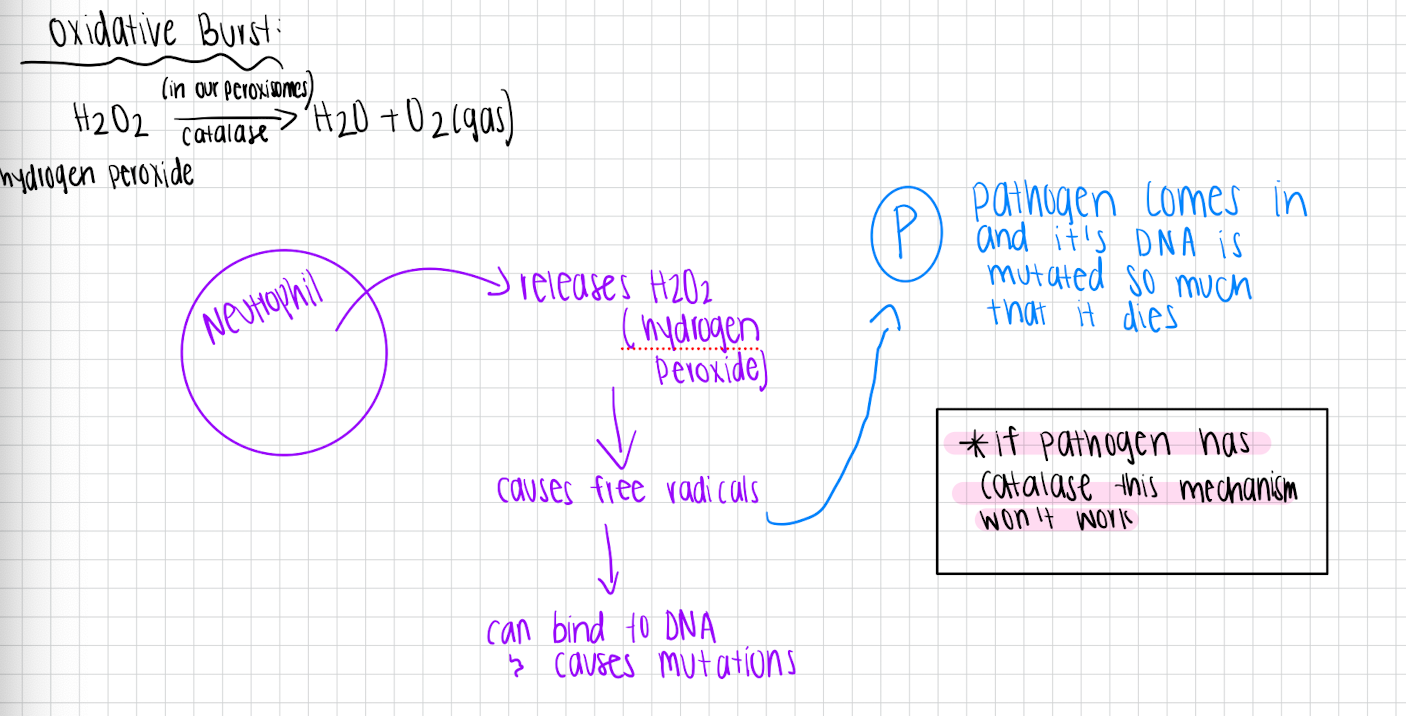
Oxidative burst
Neutrophil releases H2O2 (hydrogen peroxide) which causes free radicals → can bind to DNA and cause mutations
Pathogen will come in and it’s DNA is mutated so much that it dies
Complement proteins are ready to go as soon as
inflammation occurs
another way to attack proteins
found in serum of blood.
3 ways to turn on complement proteins
Alternative pathway (fastest)
Lectin pathway (2nd fastest)
Classical activation (slowest)
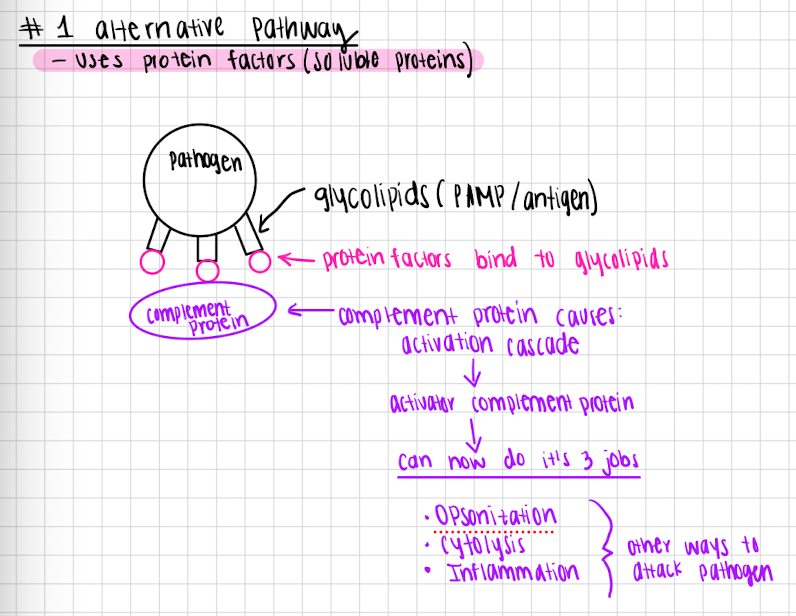
Alternative pathway (fastest)
uses protein factors
Protein factors bind to the glycolipids of the pathogen
Complement protein bind to protein factors and causes activation cascade
Complement protein is active and can do it’s jobs
Opsonization
Inflammation
Cytolysis
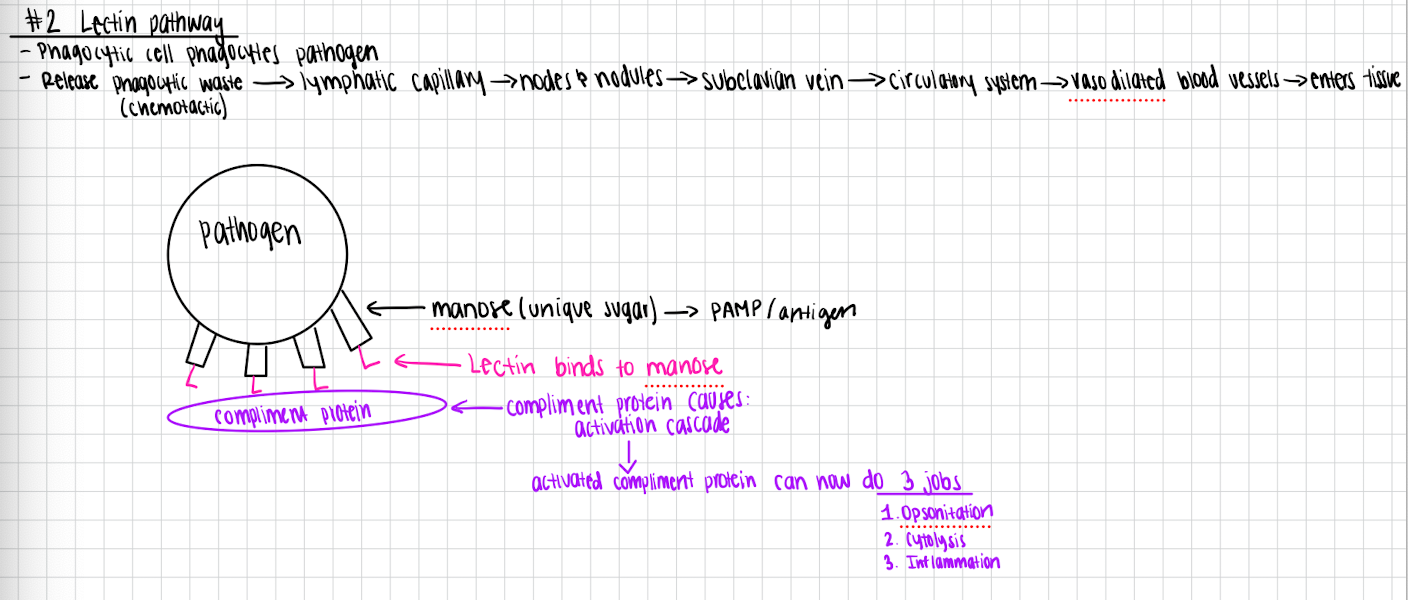
Lectin pathway
Phagocytic cell phagocytoses pathogen
Release waste → lymphatic capillary → circulatory system → makes lectin → circulatory system → vasodilated blood vessels →
Lectin binds to manose
Complement protein binds to lectin
Complement protein causes activation cascade
Activated complement protein can now do 3 jobs
Opsonization
Cytolysis
Inflammation
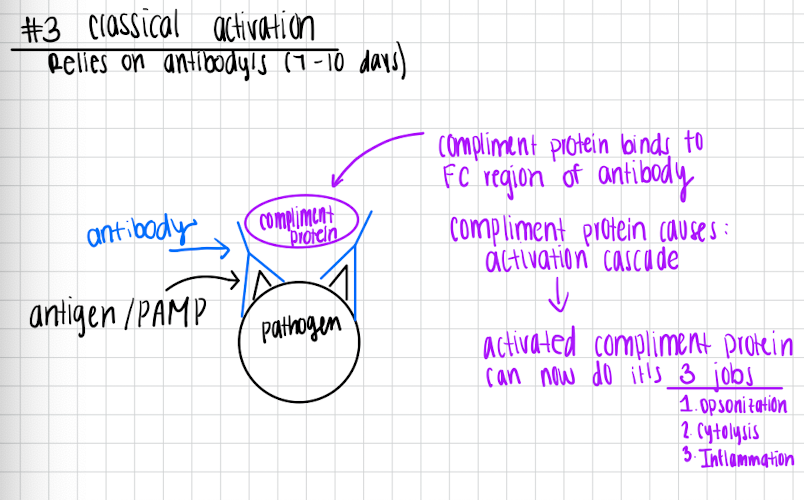
Classical activation
Relies on antibodies (7-10 days)
Pathogen has antibody’s that bind to the antigen/PAMP
Complement protein binds to the FC region of the antibody
Complement protein causes activation cascade
Activated complement protein can now do it’s 3 jobs
Opsonization
Cytolysis
Inflammation
Once compliment protein is activated by one of the 3 methods it can do all 3 of its jobs
Opsonization
Cytolysis
Inflammation
they all happen at the same time and help WBC attack pathogens
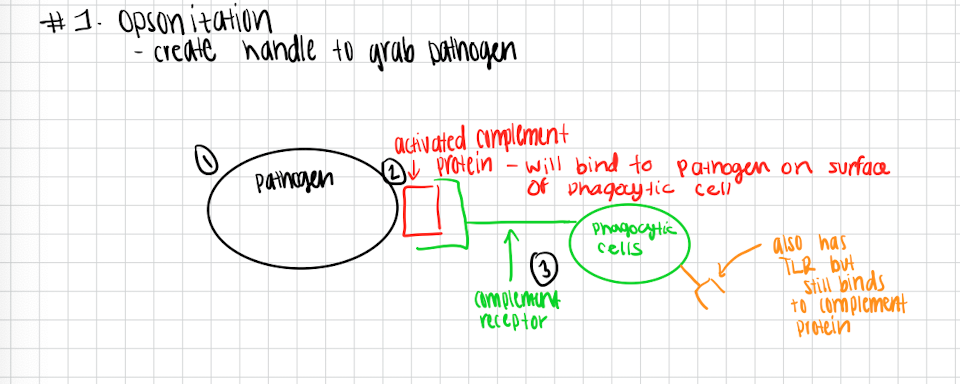
Opsonization
Creates a handle to grab pathogen
Activated complement protein will bind to the pathogen on the surface of the phagocytic cells
Complement receptor binds activated complement protein and grabs a hold of pathogen
Mast cells/ basophils are
granulocytes
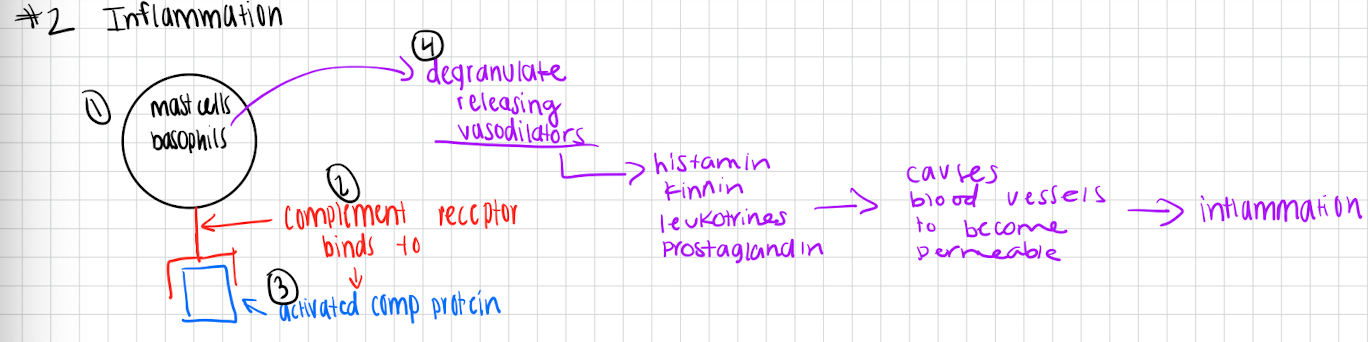
Inflammation
Mast cellls/basophils have complement receptor on surface
Complement receptor binds to activated compliment protein
This causes mast cells/basophils to degranulate releasing vasodilators (histamine, kinins, leukotrienes, prostaglandins)
This causes blood vessels to become permeable
Leads to inflammation
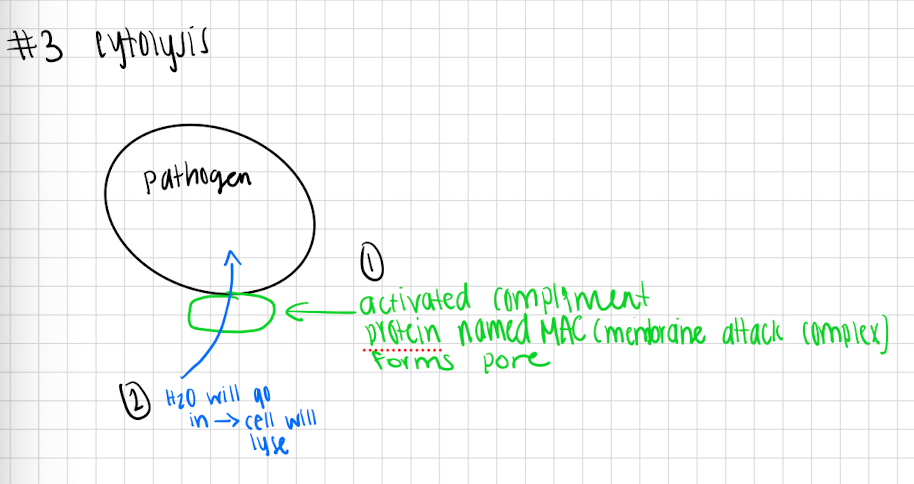
Cytolysis
Activated complement protein forms pore called MAC (membrane attack complex) in the plasma membrane of the pathogen
Since all cells are hypotonic water will be drawn inside pathogen
Cell will lyse
Some bacteria can evade complement
If pathogen has capsule, glycolipids are not exposed → complement cannot be activated
No pore → no cytolysis
If pathogen has protease → it will chew up activated compliment protein
Interferons are
cytokines
alpha, beta, gama
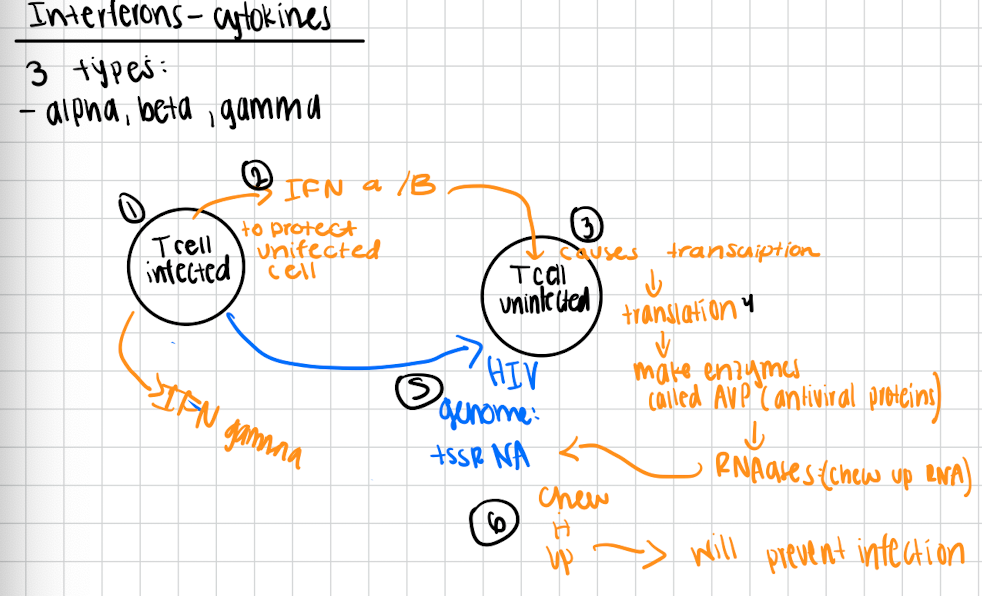
How interferons work
Viral infected cells will release interferons alpha and beta to protect neighboring viral uninfected cells
This causes viral uninfected cell to do transcription → translation → makes enzymes called AVP (antiviral proteins)
AVP are RNAses therefore they chew up the RNA genomes of the viral infection (Ex. +ssRNA genome of HIV)
They will get chewed up and it will prevent infection
Gamma interferons
activate macrophages and promote the killing of bacteria and tumor cell
Transferins
made my hepatocytes (liver) and found in serum of blood (soluble proteins)
Work by binding to iron → prevents pathogen from binding → prevents growth
always present in circulatory system
AMP’s are
released when CLR bind to PAMPs
causes pores → water rushes in → pathogen dies
have not shown ability for pathogen to become resistant to it because AMP are different from host-to-host therefore pathogen can’t evolve
Innate response
cytokines recruit phagocytes and cause inflammation
Adaptive response
Cytokines activate B and T cells to produce antibodies and attack specific pathogens
Role of inflammation in innate immunity
helps isolate pathogen, recruits immune cells, and facilities tissue repair
Antimicrobial peptides
Produced in response to TLR activation and disrupt bacterial cell
can also enhance macrophage and neutrophil activity
Innate vs Specific Adaptive responses
innate: phagocytes respond to any pathogen without specificity
adaptive: B and T cells respond to specific pathogens