Hw 6: Organic Halogens (copy)
1/44
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
45 Terms
What is the IUPAC name of the following alkyl halide?CH 3 C(CH 3 )Cl(CH 2 ) 2 CH 3
A) 2-chloro-6-methylpentane
B) 6-methyl-2-chloropentane
C) 2-methyl-6-chloropentane
D) 2-chloro-2-methylpentane
E) 2-chloro-5-isopropylpentane
D) 2-chloro-2-methylpentane
Which of the following is the best nucleophile?
A) H 2 O
B) HO -
C) HS -
D) H3S
E) all are the same
C) HS-
Which of the following is the best nucleophile?
A) H2O
B) CH4
C) NH3
D) HF
E) PH3
C) NH3
Which of the following is the best leaving group?
A) F -
B) Cl -
C) I -
D) Br -
E) H2N -
C) I -
Which of the following is the strongest base?
A) H2O
B) - OH
C) NH3
D) - NH2
E) F -
D) -NH2
The reactivity order of the following as nucleophiles is:
1. CH3S - 2. CH3O- 3. CH3OH
A) 1> 2 > 3
B) 2 > 3 > 1
C) 2 > 1 > 3
D) 3 > 2 > 1
E) 1 > 3 > 2
A

What is the mechanism of the following reaction?
A) SN1
B) SN2
C) E1
D) E2
E) both A and B
B) SN2
Which statement is true for SN2 reactions?
A) The rate of the reaction is dependent on the stability of a carbocation.
B) The rate of reaction is dependent on just the substrate.
C) The fastest reaction will occur with a tertiary halide.
D) Displacement occurs with inversion of configuration.
E) The mechanism is a two step process.
D
Which bromide reacts fastest in SN2 reactions?
A) CH3Br
B) (CH3)2CHBr
C) (CH3)3CBr
D) (CH3)3CCH2Br
E) CH3CH2Br
A) CH3Br
What is the rate-determining step in an SN1 reaction?
A) Attack of the nucleophile
B) Loss of the leaving group to form a carbocation
C) Deprotonation of the nucleophile
D) Collision between the nucleophile and substrate
E) all are the same
B
Which of the following substrates will react fastest in an SN1 reaction?
A) Methyl chloride
B) Tertiary butyl bromide
C) Primary butyl bromide
D) Secondary butyl bromide
E) The rate is equal for all substrates
B) Tertiary butyl bromide
Polyhalogenated aliphatic compounds have not been used as:
A) food preservatives
B) fire retardants
C) degreasing agents
D) insecticides
E) refrigerants
A
SN1 reactions proceed through which type of intermediate?
A) Carbanion
B) Carbocation
C) Free radical
D) Transition state
E) basic
B) Carbocation
Which is the correct rate law for an SN1 reaction?
A) Rate = k[substrate][nucleophile]
B) Rate = k[substrate]
C) K[ k[product]
D) Rate = k[nucleophile]
E) Rate = k[substrate]²
B) Rate= k[substrate]
Which of the following solvents favors an SN1 mechanism?
A) Hexane
B) diethyl ether
C) acetone
D) water
E) benzene
D) Water
Which of the following best describes the SN2 reaction mechanism?
A) One-step mechanism with backside attack
B) Elimination followed by nucleophilic attack
C) One-step mechanism with carbocation formation
D) Two-step mechanism with carbocation intermediate
E) Addition followed by nucleophilic attack
A
The slowest step of an SN1 reaction involves:
A) attack of the nucleophile on the substrate to form a pentavalent carbon.
B) breaking the bond between the carbon and the leaving group to give a carbocation.
C) combination of a nucleophile with the carbocation to give the product.
D) loss of a proton from the nucleophile to give the product.
E) none of the above.
B
The SN2 mechanism for nucleophilic substitution reactions
A) involves two steps and occurs with inversion of configuration.
B) involves one step and occurs with inversion of configuration.
C) involves two steps and occurs with racemization.
D) involves one step and occurs with retention of configuration.
E) involves one step and occurs with racemization.
B) Involve one step and occurs with inversion of configuration
The SN1 mechanism for nucleophilic substitution reactions
A) involves one step and occurs fastest with primary halides.
B) involves one step and occurs fastest with tertiary halides.
C) involves two steps and occurs fastest with tertiary halides.
D) involves two steps and occurs fastest with primary halides.
E) involves one step and occurs fastest with aromatic halides.
C) Involve two steps and occurs fastest with tertiary halides
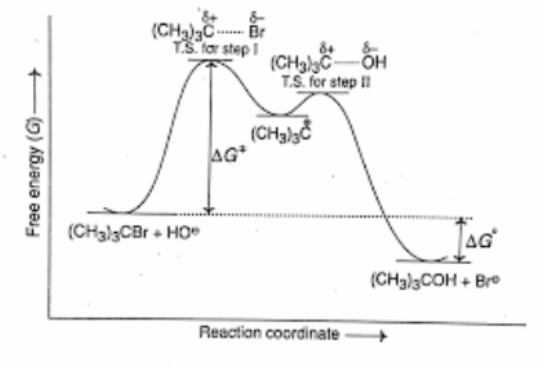
The energy-reaction diagram below is for
A) an SN2 reaction
B) an SN1 reaction
C) an E2 reaction
D) an E1 reaction
E) both SN1 and E1 reactions
F) Both SN2 and E2 reactions
B) an SN1 reaction
What is the correct rate law for an SN2 reaction?
A) Rate = k[substrate]
B) Rate = k[nucleophile]
C) Rate = k[substrate][nucleophile]
D) Rate = k[substrate]²
E) Rate = k[nucleophile]²
C) Rate= k[substrate][nucleophile]
What is the rate-determining step in an E1 reaction?
A) Loss of the base
B) Formation of the carbocation
C) Attack by the nucleophile
D) Elimination of the proton
E) Attack by the base
B) Formation of the carbocation
What is the correct rate law for an E1 reaction?
A)
B) Rate = k[base]
C) Rate = k[substrate]
D) Rate = k[base]²
E) Rate = k[substrate][base]
C) Rate= k[substrate]
When 1-bromobutane is reacted with the bulky base, potassium tert-butoxide, in tert-butyl alcohol, the major elimination product is:
A) 1-butene
B) cis-2-butene
C) trans-2-butene
D) butyl tert-butyl ether
E) butyl alcohol
A) 1-butene
Which of the following substrates is most reactive in an E1 reaction?
A) Methyl chloride
B) Tertiary butyl bromide
C) Primary butyl bromide
D) Secondary butyl bromide
E) The rate is equal for all substrates
B) Tertiary butyl bromide
In E1 reactions, what is the role of the base?
A) To attack the carbocation directly
B) To generate the leaving group
C) To remove a proton from the β-carbon
D) To replace the leaving group
E) To add a proton on the substrate
C) To remove a proton from the β-carbon
What is the defining feature of the E2 reaction mechanism?
A) Two-step mechanism with carbocation intermediate
B) Simultaneous removal of a proton and leaving group in one step
C) Involves nucleophilic substitution
D) Proceeds only with tertiary substrates
B) Simultaneous removal of a proton and leaving group in one step
What is the rate law for an E2 reaction?
A) Rate = k[substrate]
B) Rate = k[base]
C) Rate = k[substrate][base]
D) Rate = k[base]²
E) Rate = k[base][product]
C) Rate= k[substrate][base]
What alkyne is produced when a base reacts with CH3CH2CH2I?
A) 2-pentyne
B) 1-pentyne
C) 2-pentene
D) 1-pentene
E) butyne
B) 1-pentyne
Which of the following substrates reacts fastest via the E2 mechanism?
A) Primary alkyl bromide
B) Methyl bromide
C) Secondary alkyl bromide
D) Tertiary alkyl bromide
E) All of the above
D) Tertiary alkyl bromide
Which of the following is a polar aprotic solvent?
A) H2O
B) CH3CN
C) CH3OH
D) (CH3)2S=O
E) both B and D
E) both B and D
What is the rate-determining step in an SN1 reaction?
The loss of the leaving group to form a carbocation
Which type of carbocation is most stable in SN1 reactions? Give reasons.
Tertiary carbocation. It is stabilized by hyperconjugation and inductive effects from three alkyl groups
Why are primary alkyl halides not suitable for SN1?
Because Primary carbocations are too unstable to form, so SN1 cannot occur
Which type of halide (methyl, primary, secondary, tertiary) is best for SN2 and why?
Methyl and Primary halides. Because less steric hindrance allows easy backside attack in SN2
Why are bulky groups near the reaction site a problem in SN2 reactions?
Because they block the nucleophile’s backside attack, slowing or preventing SN2
What type of solvent favors SN2 reactions: polar protic or aprotic ? Explain.
Polar aprotic solvents. They don’t solvate anions strongly, keeping nucleophiles reactive
How does SN2 differ from SN1 in terms of intermediate formation?
SN2 has no intermediate, while SN1 forms a carbocation intermediate
What condition is required between the leaving group and β-hydrogen in E2?
The β-hydrogen and leaving group must be anti-periplanar (opposite sides)
Write the general rate law for an E2 reaction.
Rate= k[substrate][base]
What kind of substrate is most reactive in E2?
Tertiary Substrates
How does the strength of the base affect the E1 reaction?
Base strength has little effect on E1 rate
What is the role of the base in E2?
It removes a β-hydrogen, forming the double bond
What type of substrate (primary, secondary, tertiary) favors E1?
The Tertiary substrates
What kind of solvent is ideal for E1 reactions?
Polar protic solvents