Naming Organic Molecules
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
30 Terms
Naming Organic Molecules
"What is the first step in naming organic molecules?"
When we name molecules, we first identify the longest carbon chain. This tells us the root of the name
"After identifying the longest carbon chain, what is the next step in naming organic molecules?"
Once we’ve identified the longest carbon chain to write the root, we need to identify the functional groups and substituents
In this example the molecule contains three methyl groups and a ketone
"What is the next step after identifying the functional groups and substituents in naming organic molecules?"
Once we’ve identified the functional groups and substituents, we need to identify the highest priority functional group
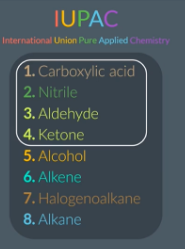
highest priority functional group
IUPAC
In the exam you don’t need to be remember the entire list just remember that these 4 take higher priority.
can nice ants knit
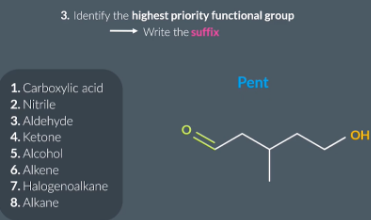
"After identifying the highest priority functional group, what is the next step in naming organic molecules?"
Once we’ve identified the highest priority functional group, we write the suffix.
The highest priority functional group is an aldehyde so the suffix is anal
highest priority functional groups + suffix
"When writing the suffix in naming organic molecules, which groups are not considered?"
When we write the suffix we don’t take into account halogenoalkanes or branched alkanes because they are always names in the prefix
"When numbering the carbons in a molecule, what is the goal?"
"How do we number the carbons if the highest priority functional group is in the centre of the molecule?"
If the highest priority functional group is in the centre of the molecule, we number the carbons so that the second priority functional group gets the lowest position number.
"How do we number the carbons if there are two functional groups of equal priority?"
If we have two groups of equal priority, we number the carbons whichever way gives us the lowest individual position number.
"How do we include substituents in a molecule's name?"
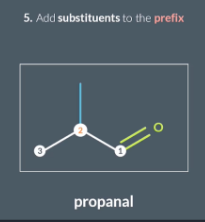
Once we’ve numbered the carbons, we add any remaining substituents into the prefix , including their positon number .
E.g this molecule contains a methyl group on the 2nd carbon so we use the prefix methyl and add 2 before it
"How do we name alcohols as the highest priority functional group?"
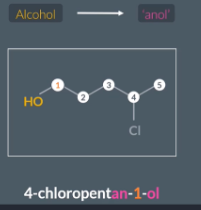
When the alcohol is the highest priority functional group, we write the suffix ‘anol’
Alcohols are high priority that halogenoalkanes so we write the prefix anol. With position number ‘an - position number - ol’
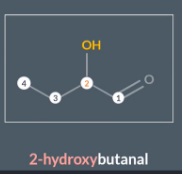
"How do we name alcohols when they are not the highest priority functional group?"
When the alcohol isn’t the highest priority functional group, we write the prefix ‘hydroxy’
Aldehydes are higher priority than alcohols so we include the alcohol in the prefix with the position number before the hydroxyl: position number - hydroxyl
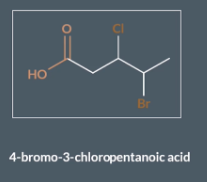
"How do we order multiple substituents when naming a molecule?"
When there are multiple substituents included in the prefix, we write the substituents in alphabetical order.
The molecule contains 5 carbons so ‘pent’. It contains a ‘COOH’ so it's a carboxylic acid which means it ends in ‘anoic acid’. The prefix contains 3 - chloro and 2-bromo
We order these alphabetically 2-bromo-3 chloro. And then add pentanoic acid. SO the name is 2-bromo-3chloro-pentanoic acid
multiple identical substituents
multipliers
"How do we name a molecule with multiple identical substituents?"
When a molecule has multiple idenitcal substituents we include multiples and we write the positon numbers of each individual subsitiuent
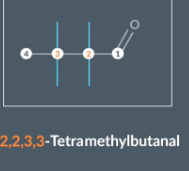
"How do we name a molecule with four identical methyl groups?"
There are 4 methyl groups so we include the prefix tetra. 2 of the methyl groups are on carbon 2 and 2 are on carbon 3 so we write 2,2,3,3
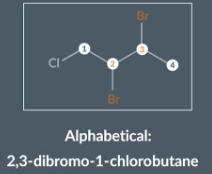
"How do we order substituents alphabetically when some are identical and some are different?"
IF a molecule contains multiple substituent where some are different and some are the same we don't take the multiplier into account when we order the substituents alphabetically.
We look at ‘bromo’ rather than ‘dibromo’ which explains why ‘dibromo’ comes before ‘chloro’ in the molecules name
how to name molecules
"How do we name alkenes, including double bond position, substituents, and stereoisomers?"
In alkenes the longest carbon chain has to include a double bond. When naming alkenes we need to include the position of the double bond and subsituents. If alkenes have steroisiomer we need to include the subsituent E/Z in front of the name
When we name molecules with alkenes and alcohols, we
change ‘an’ to ‘en’ so ‘enol’ instead of ‘anol’ + add the lowest position number of the cabron before it + add either E or Z if the alkene has stereoisomers.
"Naming alkenes with alcohols and E/Z isomerism?"
Alkenes with alcohols:
E/Z isomer -- longest carbon chain prefix - lowest positon number of double bond - ‘en’ - potion number of alcohol - ‘ol’
Name this molecule
6 carbons in the longest chain so ‘hex’. Double bond is located on the 4th carbon. It is an alkene and has an OH group on the first carbon so ‘en-1-ol’. It's a Z isomer (OH is on same side as double bond).
SO Z-hex-4-en-1-ol
"Naming molecules with alkenes and carbonyl groups?"
Change suffix: Replace ‘-anoic’ with ‘-enoic’ for carboxylic acids, and ‘-anal’ with ‘-enal’ for aldehydes when alkenes are involved.
Position number: Include the lowest position number for the double bond if necessary.
E/Z isomerism: If the alkene has stereoisomerism, include either E or Z in front of the name.
"Naming complex cyclic molecules?"
Root: Follow the same naming procedure, but add 'cyclo' before the root name.
Position numbers: Only include position numbers if the molecule contains more than one functional group or substituent.
Example: If there are 2 chlorine substituents on carbons 1 and 3, the name would be 1,3-dichlorocyclohexene.
for example name this moelcule
E.g this molecule has 4 carbon so we write the root ‘but’. Because they form ring we add ‘cyclo’ before it. We dont include halogens in the suffix so we add the root ‘ane’ (for alkane). This molecule only contains one bromine atom so we just write the prefix ‘bromo’
SO the name of the molecule is bromocyclobutance
name this molecule
This molecule has 5 carbon so we write the root ‘pent’. Its an alkene so a double bond is one the first carbon so pent-1-ene.There is a methyl gorup on the 3rd carbon so the name is 3-methylcyclopent-1-ene
"Naming dicarboxylic acids?"
Write the full name of the longest carbon chain.
Use the suffix ‘dioc’ acid.
No need for position numbers because carboxylic acids are always at the end of the chain.
Example: For a 4-carbon chain with two carboxylic acid groups, the name would be butanedioic acid.
Drawing complex molecules
When we draw complex molecules, we use the root to draw the longest carbon chain.
Next, we number the carbons left to right.
Next, we use the suffix to add the highest priority functional group.
Finally, we use the prefix to add the remaining substituents.
"Why isn't IUPAC always practical for naming?"
IUPAC nomenclature ensures consistency among chemists, but sometimes it's not practical.
Some IUPAC names can be very long (e.g., "titanium dioxide" vs. its IUPAC name).
In such cases, shorter names are often used for convenience (e.g., calling titanium "titan").
Common names are easier and quicker for communication.