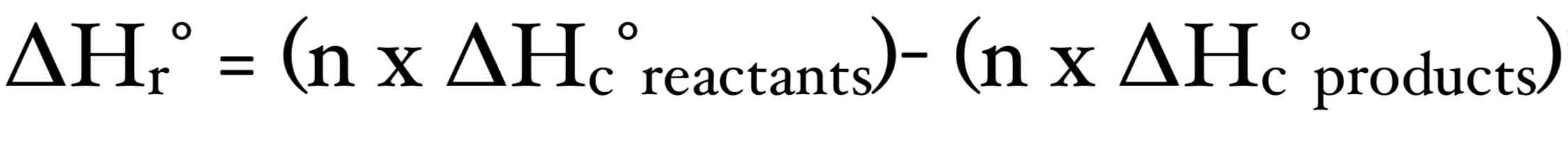Hess’ Law
1/5
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
6 Terms
Define Hess‘ Law
enthalpy change for any chemical reaction will be the same regardless of the ‘path’ taken as long as the starting conditions and final conditions, and reactants and products, are the same
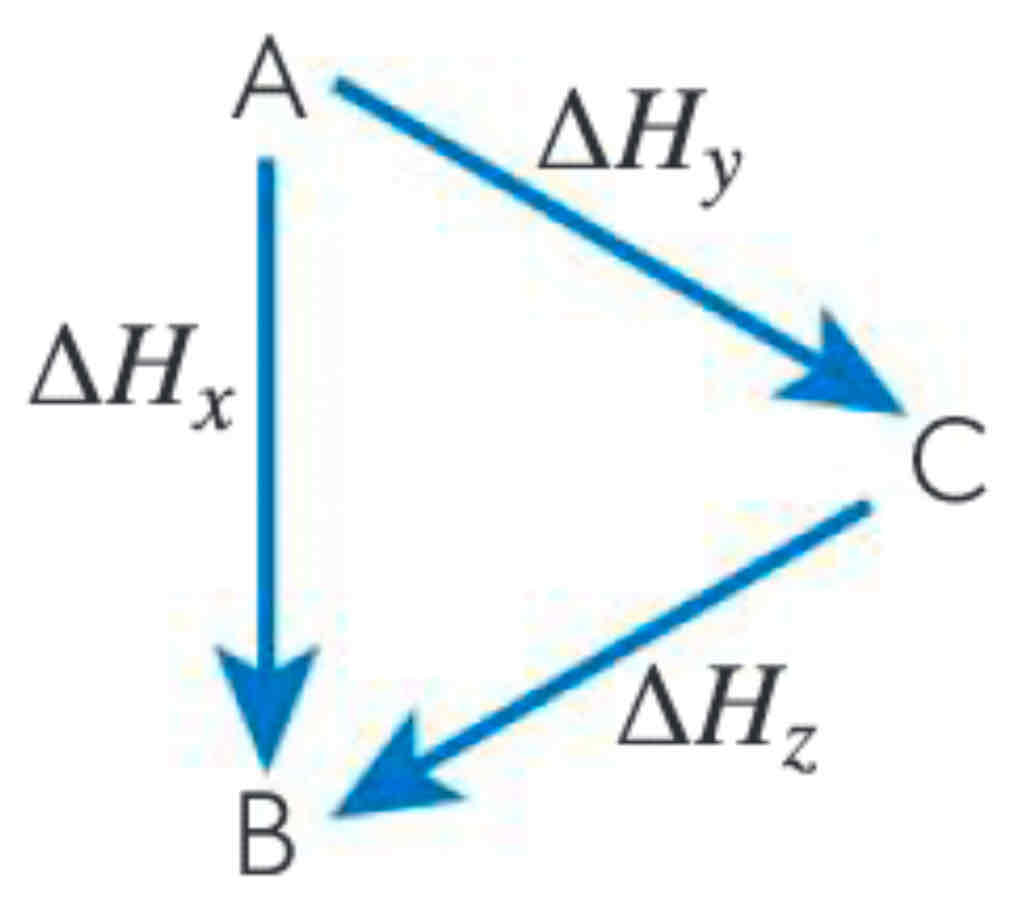
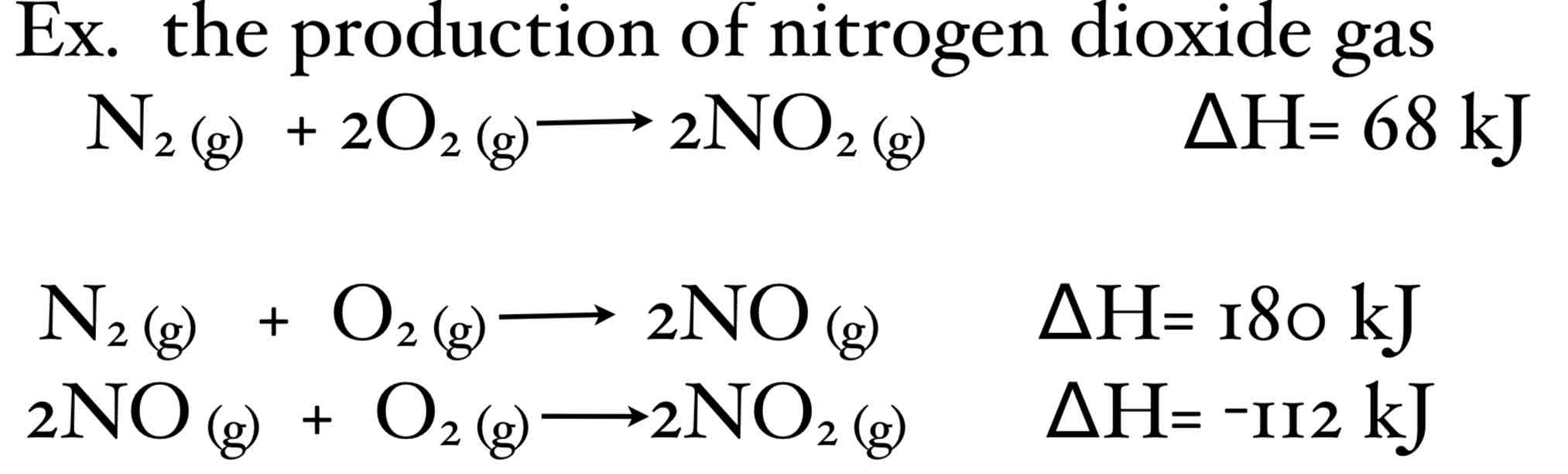
How would you use Hess’ Law in this instance?
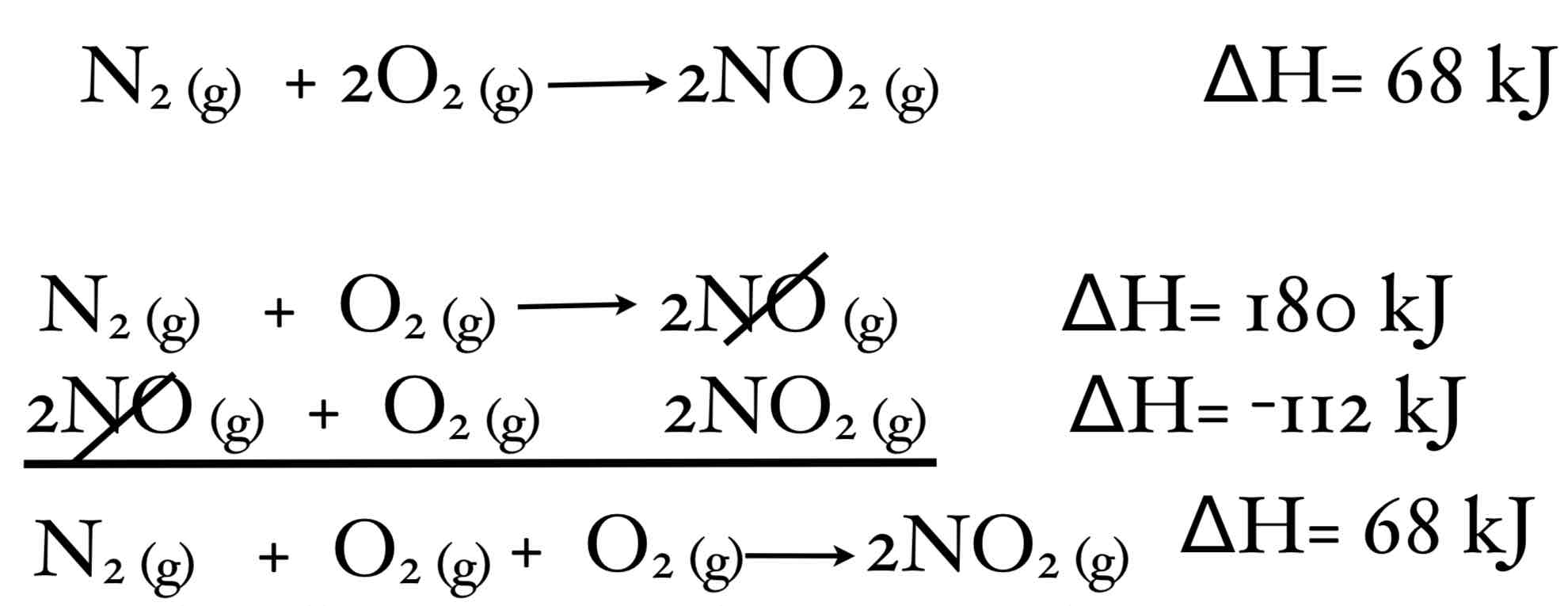
What are the rules of Enthalpy changes?
∆H must accord with energy flow
if flow reversed, then flip sign
∆H is proportional to the number of moles, so if moles are manipulated, then ∆H must be equally manipulated
Tell me about Standard Enthalpy Changes of Reactions
occur under standard conditions
The reactions are either formation, ∆Hf, or combustion, ∆Hc
indicate stability of substance and used to calculate enthalpy changes of all reactions (real and hypothetical)
Tell me about Enthalpy Changes of Formation Reactions
denoted by ∆Hf
∆Hf of an element at standard state is (SATP) is zero

Tell me about Enthalpy Changes of Combustion Reactions
one mole of a substance is completely burned in oxygen
denoted by ∆Hc
products are carbon dioxide and water
can use fractions to balance