Oncogenes & Tumour Suppressor Genes
1/39
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
40 Terms
How many different types of cancer
> 100
Cancer cells are heterogenous. How do they vary?
Cure rate & treatment response
Genetic makeup between cells within the same tumour
Presence of cycling vs. resting cells
Ability to spread (metastasis)
Define gene
The basic unit of heredity passed from parent to child
On what 4 levels can we regulate genes
DNA to RNA:
Transcription
RNA
Splicing
mRNA stability
RNA to Protein
Translation
Protein
Protein stability
Post translational modifications
Cellular localization
Cancers are genetic and epigenetic diseases. Which part (genetic/epigenetic) can be reversed
Epigenetic change can eb reversed
(In genetic change, it is irreversibly fixed in the genetic material of the tumour cell)
Give 2 examples of genetic changed
Point mutations - Single nucleotide base changes
Gross chromosomal rearrangements
Do point mutations affect DNA/RNA/Protein
All 3 - Present in the DNA, transcribed into RNA – can result in changes to the encoded protein
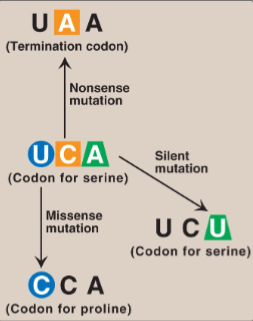
Name & explain 3 examples of types of point mutations
Nonsense mutation: Altered codon encodes a termination codon → shortened protein
Missense mutation: Altered codon encodes a different amino acid → non-functional / hyperactive protein
Silent mutation: Altered codon encodes for the same amino acid
What is the consequence of Gross chromosomal rearrangements
increased/decreased copy number and gene expression
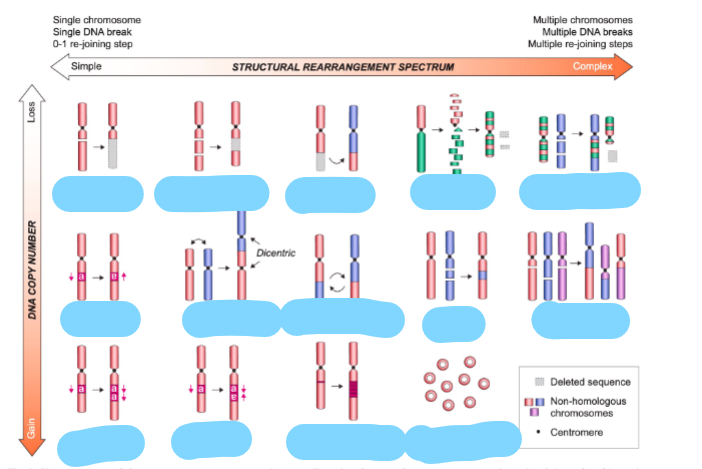
Name these chromosome rearrangements
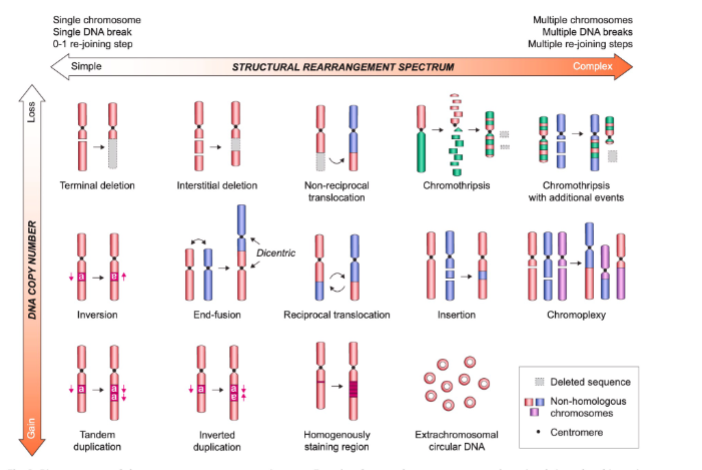
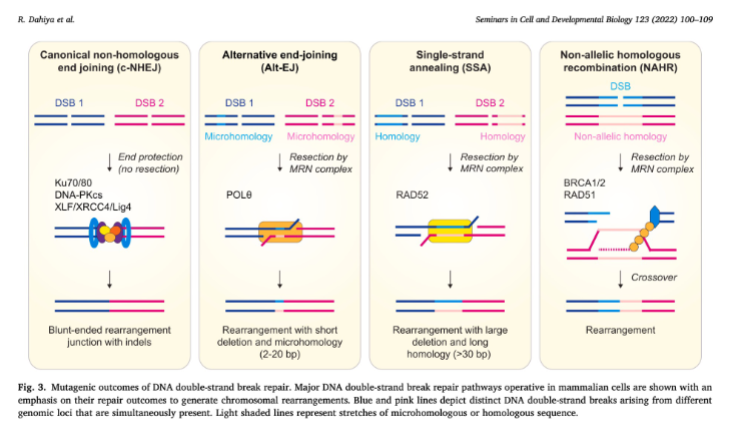
Give 4 mutagenic outcomes of DNA double strand break repair
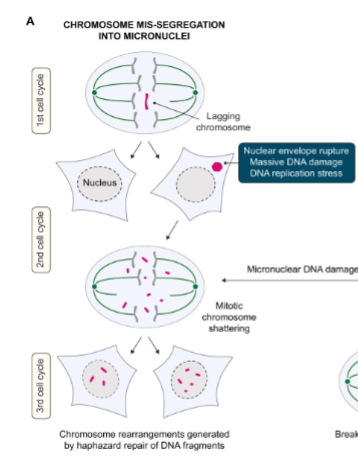
Explain how micronuclei form & what goes wrong with them
A chromosome gets left behind during cell division → forms a micronucleus.
Micronucleus is defective → its envelope often breaks.
DNA inside gets massively damaged.
In the next divisions, the shattered DNA gets randomly stitched back together.
Result: chromosome rearrangements (very unstable, cancer-promoting).
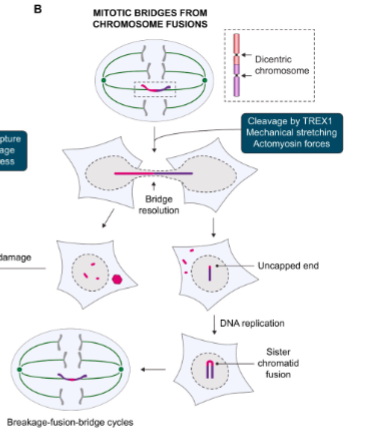
Explain how mitotic bridges form & what goes wrong with them
Two chromosome ends fuse → form a dicentric chromosome (two centromeres).
During division, the two centromeres get pulled apart → forms a DNA bridge.
The bridge breaks due to mechanical stress or enzymes like TREX1.
Broken ends are uncapped → they fuse again after DNA replication.
This cycle repeats (breakage–fusion–bridge cycle) → causes ongoing DNA rearrangements.
Give 2 examples of epigenetic changes that can occur in cancer
DNA methylation
Histone modifications
(both affect gene expression)
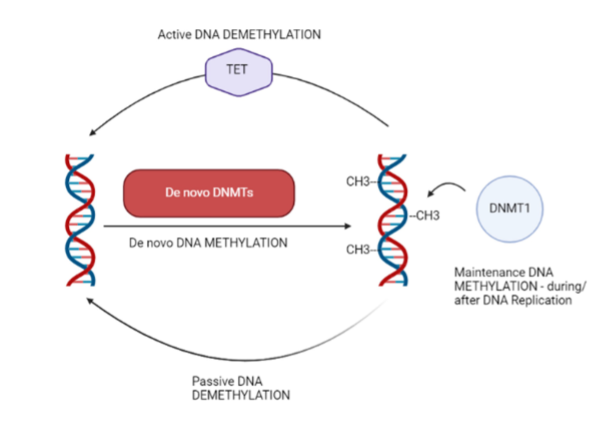
Process of DNA methylation
DNA methylation is a biochemical process where the 5th carbon present in cytosine is enzymatically methylated
Enzymes catalysing DNA methylation are…
DNMT1
DNMT3A
DNMT3B
DNA can be passive or active with the use of what
TET
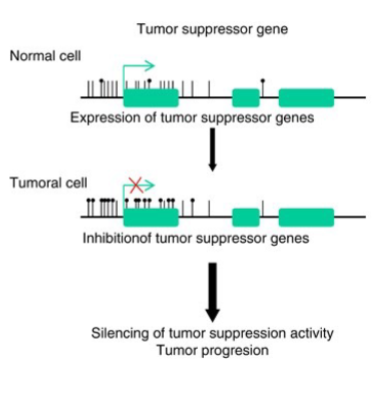
How ca DNA methylation affect tumour suppressor genes
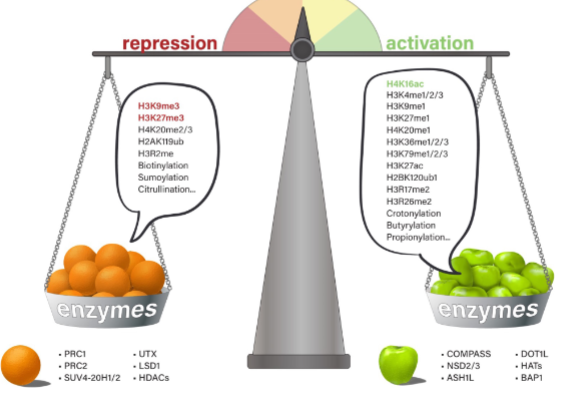
Give 3 examples of histone modifications:
2 repressive modifications
1 activation modification
Repressive: H3K9me3 & H3K27me3
Activation: H4K16ac
What creates & removes histone modifications
Enzymes
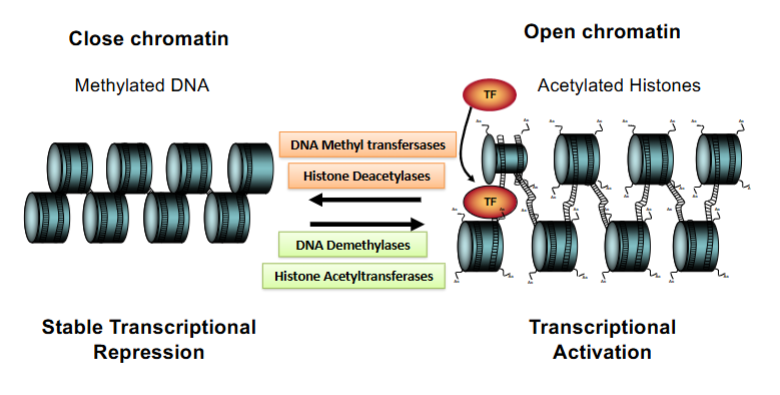
What enzymes are in charge of changing the chromatin conformation from closed → open → closed
What therapy can be used for genetic changes in cancer cells
Gene therapy
What therapy can be used forepi genetic changes in cancer cells
Targeting enzymes with small molecules → reverse epigenetic change
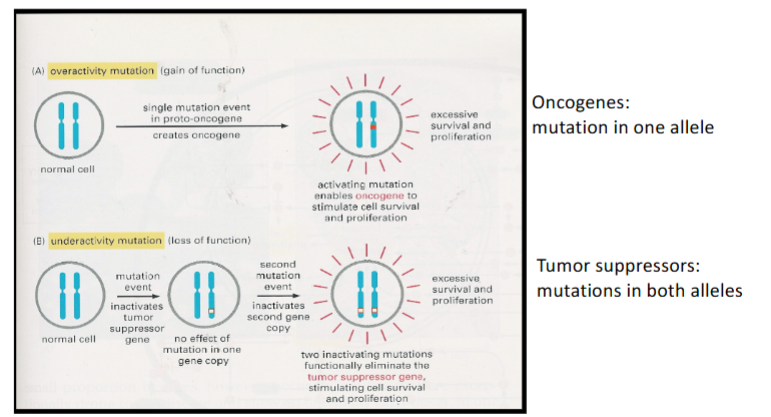
What are the 2 types of genes altered in cancer cells
Oncogenes
Tumour suppressor genes
Give 3 examples of oncogenes
myc, ras, abl
Give 3 examples of Tumour suppressor genes (TSG)
RB, p53, BRCA1, BRCA2
Are oncogenes & tumour suppressor genes dominant/recessive
Oncogenes act in a dominant manner
Tumour suppressors are recessive
Difference between Ras GDP & Ras GTP
RAS-GDP = OFF. When RAS is bound to GDP, it is inactive → no growth signal.
RAS-GTP = ON. When RAS binds GTP, it turns on → sends a strong “grow and divide” signal.
Why is Ras an important molecule clinically
Mutations in Ras and in the Ras pathway are very common in human cancers
What is a change that can occur which would make Ras an oncogene
If it becomes a mutant protein that lacks GTPase activity, it is active [‘on’] all the time
Burkitt’s lymphoma is cancer of what
Cancer of lymphocytes
Burkitt’s lymphoma is common where in the world
certain parts of Africa
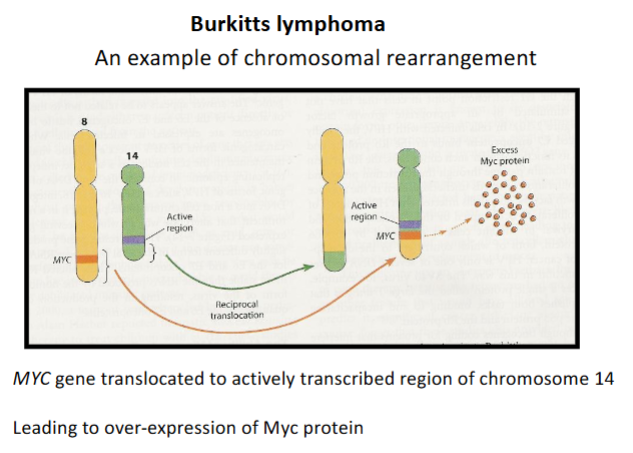
What gene is involved in Burkitt’s lymphoma & what mutation occurs to it
c-MYC gene translocated from chromosome 8 to chr. 14
What is the result of c-MYC gene translocation in Burkitt’s lymphoma
Enhanced production of Myc protein → Stimulates cell proliferation → tumour formation
Myc regulates proliferation through what molecules
↑ CDKs - cyclin dependent kinases
↓ Inhibits p15
↓ Inhibits p21
What is Li-Fraumeni syndrome
A rare cancer-prone syndrome where people are born with a germline mutated copy (allele) of p53 so their risk of cancer is much higher because they only need 1 somatic mutation in the other copy (allele) of p53 gene.
Leads to early onset of variety of cancers – of blood, breast, bone, lung and skin
What does p53 normally do
It is a Transcription factor → Mediates Cellular stress signals
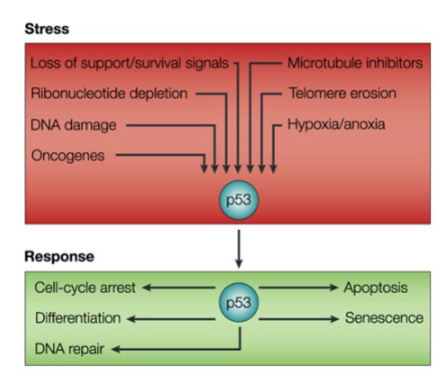
How does a p53 mutation lead to cancer
Mutation in p53 leads to loss of ability to arrest cell cycle progression after DNA damage
Cell continues to divide in the presence of DNA damage
Increase in mutations in genome – genome instability
Also become resistant to some chemotherapeutic agents
True/False: Multiple lesions are required to cause most cancers
True
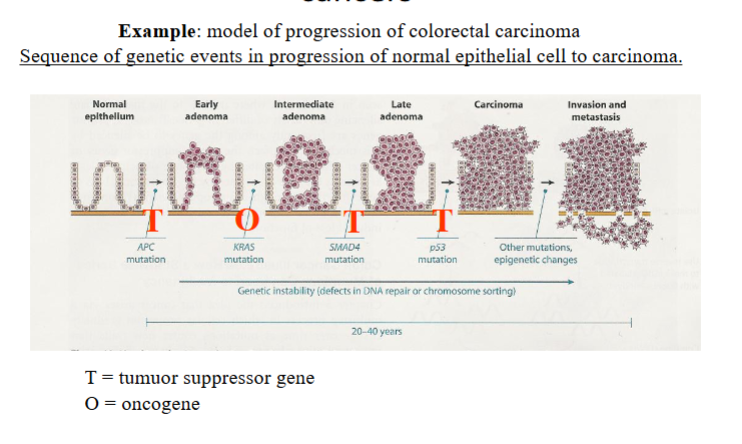
What shows that Cancer genomes are unstable
Deep sequencing approches of genomes have revealed high degree of intratumural heterogeneity