EBP (2) (copy)
1/40
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
41 Terms
NNH
The number of people who when treated results in one additional harmful outcome.
1/Absolute risk difference. → must be to nearest whole number
NNT
Number of patients who must be treated with new treatment to achieve one more success than on old treatment.
1/ Absolute risk reduction → must be rounded to nearest whole number
Incidence rate
Which keeps into account the changes in population

What is Prevalance?
ALWAYS between 0 – 1

Types of prevelance
Point prevalence - The proportion of people who have a disease at a specific point in time.
Period prevalence - The number of people who have the disease in a time divided by the population at the mid-point of the period.
What can affect prevalence values?
Changes in incidence
Changes in disease duration
Intervention programmes
Changing classifications
How do we measure the affect of disease?
Absolute measures e.g., NNT/NNH or absolute risk
Relative measures such as risk ratio
What are clinical trials?
They are designed to determine efficacy and safety by collecting data on drugs and devices. They are the first-time new drugs are tested in patients.
Types of blinding in trials
Double blind
Ideal
Protects against information bias
Neither patient or physician/researcher knows the treatment being taken
Single blind
Sometimes necessary.
Patient or assessing physician does not know which treatment is being taken.
Type of data collected in trials
Baseline data collected prior to start of treatment.
Efficacy.
Safety data including serious and non-serious adverse events or toxicities.
Follow-up status.
What are endpoints in trials?
Clinical endpoints
Reflect survival or symptomatic status of patient.
Surrogate endpoints
Associated with clinical endpoints but do not directly reflect survival or clinical status of the subject (comes before clinical end point)
Examples: In Diabetes the surrogate endpoint may be the HbA1c or Serum glucose
Main difficulty is that they’re not in the pathway of the effect of intervention
PPA
Per protocol Analysis
Includes only those events that occur while participant is complying with intervention.
May be performed in addition to ITT.
Vulnerable to bias.
Self-selected healthy population?
ITT
Intention to treat
All events included regardless of adherence.
All outcomes must be ascertained regardless of whether the treatment has been discontinued.
What disadvantages to ITT analysis?
Subjects who discontinue no longer receive the benefit from the treatment.
Adverse events occurring after treatment discontinuation bias analysis.
Study may lack power (and credibility) where nonadherence is significant.
Nonadherence can be beneficial in non-inferiority or equivalence trials.
Types of Observational Studies
Descriptive studies:
Case reports
Case series
Secular trend analysis (no comparative)
Cross-sectional study
Drug utilisation
Analytical Studies:
Cohort studies
Case-control study
Pros and Cons of Observational Studies
Pros:
nonexperimental so no harm to patient
can select subjects included.
Con:
No control over exposure assigned.
Confounding can occur.
How are cohort studies conducted?
From population you select your cohort of interest.
Can either join at the start (this is a fixed cohort) or join at any time during the period (this is a dynamic cohort).
Need to be free of outcome under study at the start.
Collect data and identify the outcomes of the study.
Need good definition of exposure.
Determine the effects of rare exposures.
What is the importance of exposure in observational studies?
The exposure of interest needs to be clearly defined.
Misclassification of exposure may lead to an underestimate or overestimate of association between exposure and outcome.
What is the pros and cons of cohort studies?
Strengths
Efficient for rare exposures.
Multiple effects can be studied.
Studies are less prone to bias.
Can calculate incidence rates, relative risks.
Temporal relationship easier to establish.
Limitations
Can have difficulties with loss to follow-up.
May have problems with bias.
What are factors we need to consider when finding associations?
Strength of association
Consistency
Specificity
Temporality
Biologic gradient
Biologic plausibility
Coherence
Experimental evidence
Analogy
Cases vs Controls
Cases are those with the outcome of interest.
Controls are those without the outcome of interest (disease of interest) but otherwise comparable to the cases found.
Advantages and Disadvantages of Community Controls
Advantages:
Might be more representative.
Disadvantages:
Harder to recruit (less interest in study?)
More issues with recall bias?
Advantages and Disadvantages of Hospital Controls
Advantages:
easy to recruit.
Disadvantages:
might not come from the same population.
might be more likely to have risk factors such as smoking / alcohol / poor diet than the general population.
What is the Odds Ratio calculation?
Comparison of ODDs
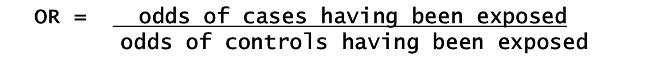
What is ODDs?
ODDS are the probability of an event happening divided by the probability that it does not happen.
If the probability of an event = p then the ODDS of that event are p/(1-p)
What do the results of Odds Ratios mean?

What is bias?
Systematic error in the data.
This results in the risk estimate being pulled away from its true value.
Cannot be eliminated at point of analysis
Types of bias
Information bias:
Interviewer bias → interviewer knowing someone is a caser and probing more for demand characteristics to involve them in the study
Misclassification of exposure.
Recall bias.
Measurement bias.
Selection bias:
Who volunteers?
Selecting controls who aren’t controls.
Selecting cases who aren’t cases.
What are the types of misclassifications?
Non-differential:
Misclassification to same degree for all groups.
Non-differential misclassification: underestimate effect (for two exposure categories).
Differential:
Misclassification different between groups.
Differential misclassification can bias result in either direction.
What is confounding?
A variable that affects the two being studied causing the results to be distorted and not reflect the actual relationship of the study
A confounder is a risk factor for the disease and is correlated with the exposure independent of disease.
Confounder must be associated with exposure and with outcome INDEPENDENTLY.
Effect of confounding: can underestimate or overestimate effect.
A factor’s ability to be a confounder is entirely dependent on whether it is unevenly distributed between the study groups.
The indication for prescription is one of the most important factors to consider when evaluating medication exposures
Chanelling
Where a drug is prescribed to a group of patients because of:
a characteristic of the drug.
a characteristic of that group of patients.
It is a new drug.
How can we control confounding?
Randomisation - Evenly distribute confounders between study groups
Restriction - Gives much more control but cannot study variation between levels of that factor
Matching - Each case is paired with a control subject(s) for specified constraints e.g. confounding factor(s) HOWEVER:
Can be difficult to find a match.
Risk of over-matching.
Can’t determine the association with a factor that is used for matching.
Only controls for the criteria that have been matched on.
Stratified analysis - Separating out factors so any mixture of their effect is removed. This can be used for one or two factors.
Multivariate analysis - Considers several factors simultaneously.
Effect modifiers
Effect modification occurs when the effect of the exposure is different in different groups of the population.
A factor that modifies the effect of a putative causal factor under study.
There is no average ‘true value’.
It is important to identify where these may occur so that you can know where the risk estimate is pulled.
Pharmacovigilance
the detection, assessment and prevention of adverse drug effects in humans
Pharmacoepidemiology
The principles of chronic disease epidemiology applied to the area of clinical pharmacology e.g.
How is the drug used?
What are the predictors of use?
What are the outcomes?
Yellow Card Scheme
A Pharmacovigilance system used in the UK used to monitor the safety of medicines and vaccines.
It allows healthcare professionals, patients, and the general public to report suspected adverse drug reactions (ADRs) and other safety concerns and is managed by the MHRA
You
Adverse Drug Reactions (ADRs): Any undesirable effects experienced after using a medication, particularly those not listed in the product information.
Medication Errors: Mistakes in prescribing, dispensing, or administering medication, even if they do not result in harm.
Defective Medicines: Issues with the quality, packaging, or labeling of a medication.
Counterfeit Medicines: Suspected fake or substandard drugs.
Data Collection and Analysis:
The MHRA collects and analyzes the data from Yellow Card reports to identify new safety issues or patterns of adverse reactions. This helps in updating safety information and ensuring that the benefits of medicines outweigh their risks.
Impact on Public Health:
Regulatory Actions: The data from the Yellow Card Scheme can lead to regulatory actions, such as updating the safety warnings on a drug’s label, restricting its use, or even withdrawing it from the market.
Communication: MHRA may communicate new safety information to healthcare professionals and the public through safety alerts, bulletins, and updates to product information.
Education: The scheme helps in educating healthcare providers and patients about the potential risks associated with medications and the importance of reporting ADRs.
Importance of the Yellow Card Scheme
Enhanced Drug Safety:
By providing a platform for reporting ADRs and other issues, the Yellow Card Scheme enhances drug safety and helps in early detection of potential problems.
Post-Marketing Surveillance:
It serves as an essential tool for post-marketing surveillance of medicines, complementing the data obtained from clinical trials, which might not always reveal all possible side effects due to limited participant numbers and duration.
Patient Involvement:
Involving patients in reporting ADRs empowers them to take an active role in their healthcare and contributes to a more comprehensive safety monitoring system.
Continuous Improvement:
The scheme fosters continuous improvement in the safety profile of medications by ensuring that new risks are promptly identified and managed.
Who Can Report for the Yellow Card Scheme?
Both healthcare professionals (such as doctors, pharmacists, and nurses) and the general public (patients and caregivers) can submit reports.
What can be reported on the YCS?
Adverse Drug Reactions (ADRs)
Medication Errors
Defective Medicines
Counterfeit Medicines
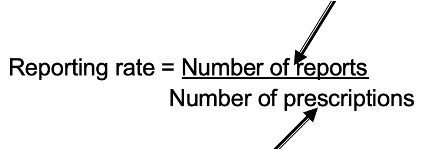
Reporting Rate
Prevalence
Proportion of a population that has a specific characteristic or condition at a given time
RCTs
Randomized Controlled Trials (RCTs) are considered the gold standard in clinical research because they minimize bias by randomly assigning participants to either the treatment group or the control group. In pharmacy, RCTs are essential for determining the efficacy and safety of new drugs