GCSE Chemistry Revision "Exothermic and Endothermic Reactions
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
What happens during exothermic reactions?
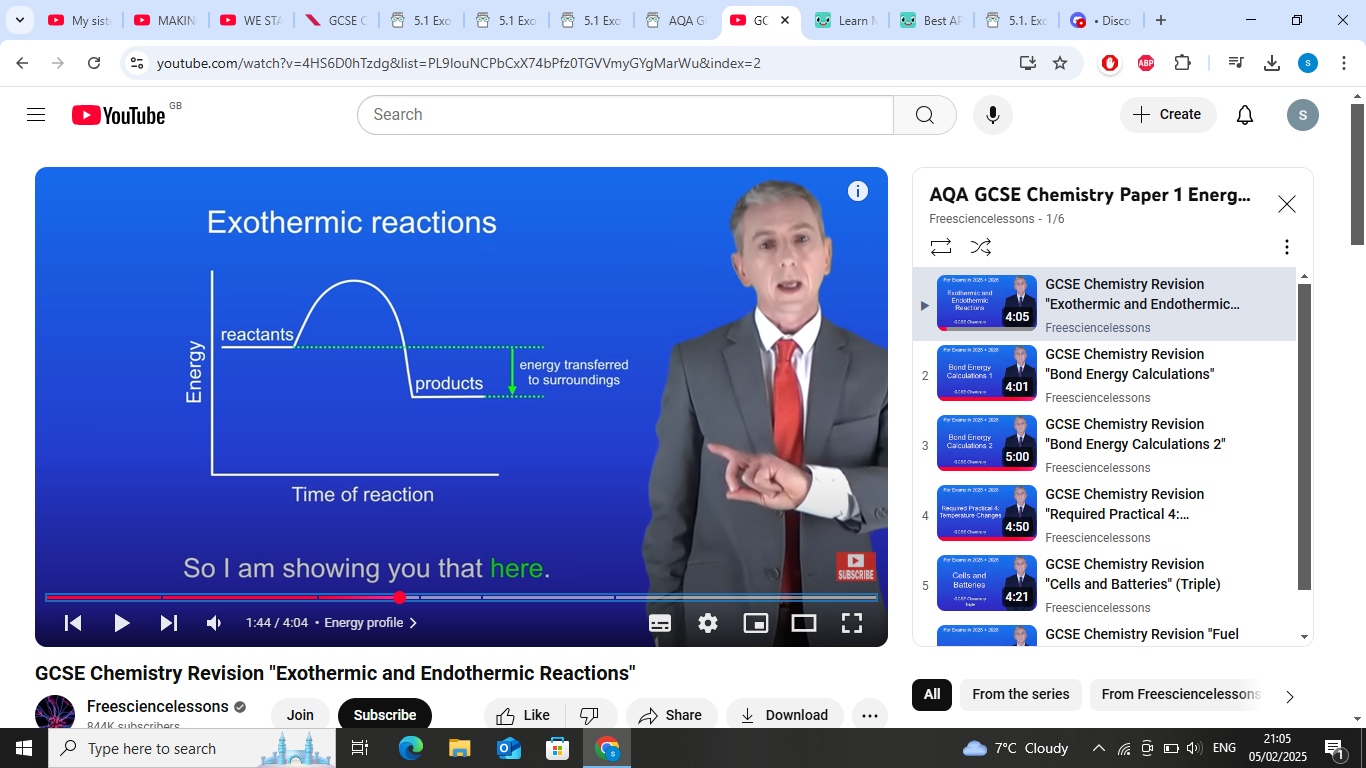
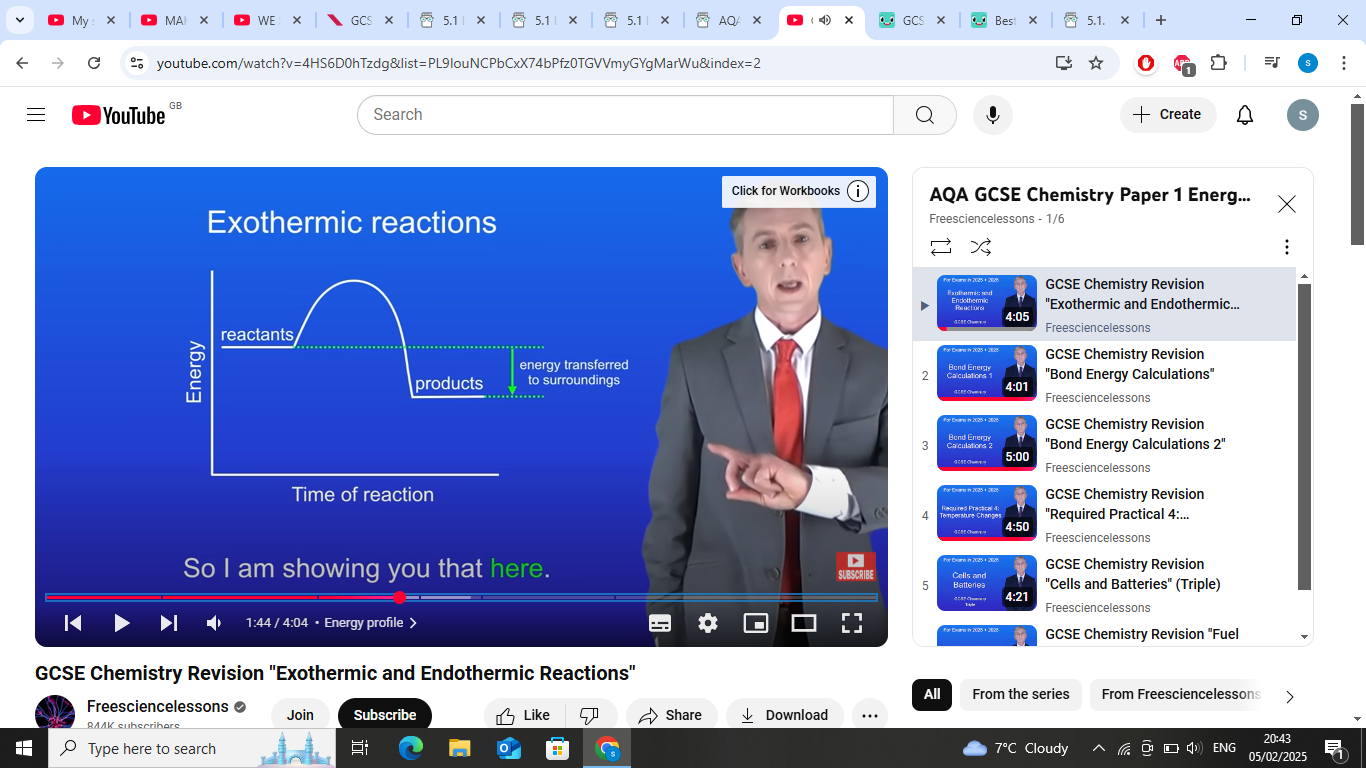
exothermic-Energy is released into the surroundings.
in the exothermic reaction graph,products less than ___ why?
exothermic reaction graph- products have less energy than reactants as energys transferred from the reaction to the surroundings
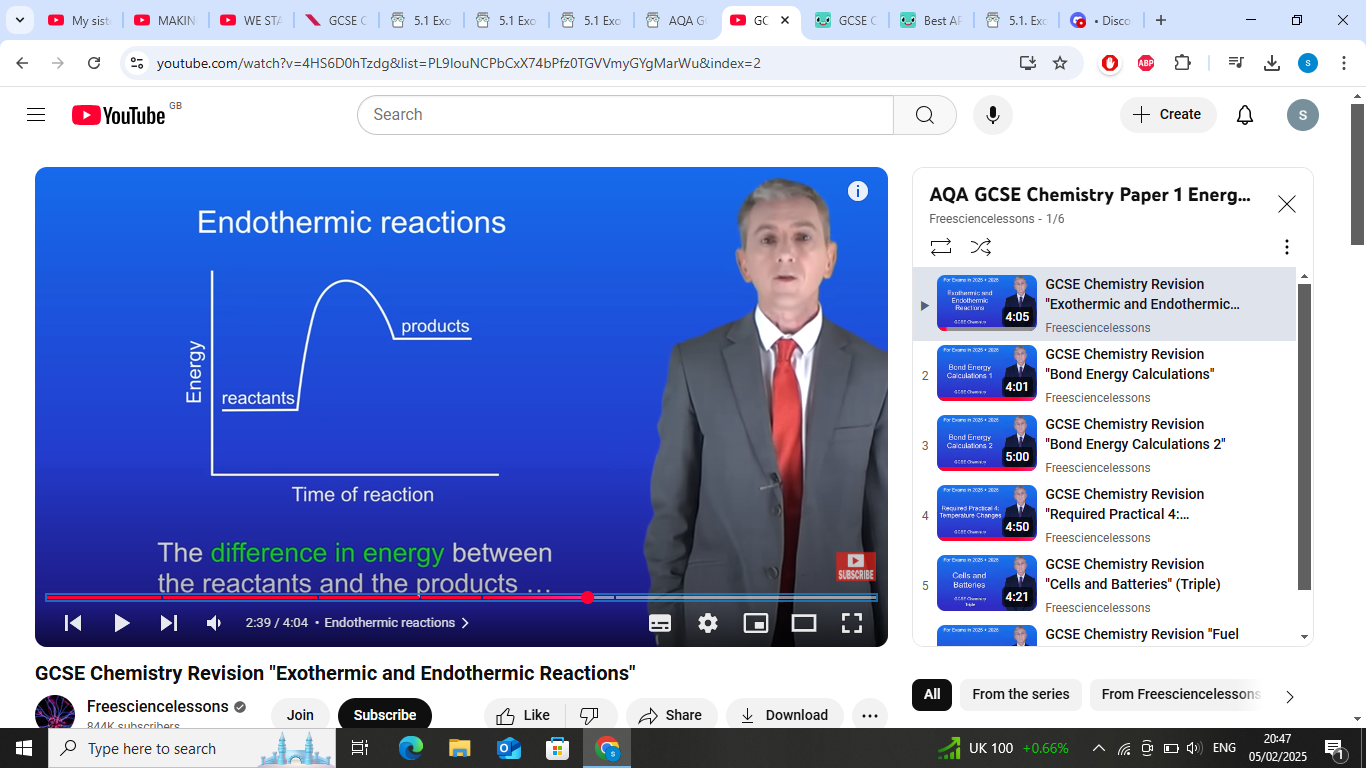
what does reactants to products in endothermic tell us in the graph?
reactants to products in endothermic tell us energy taken in from surroundings
What effect do exothermic reactions have on the temperature of their surroundings?
the temperature of their surroundings increase
whats reactants to products in exothermic reactions?
tells us the energys released into the surroundings (other arrow)
energy transferred to surroundings in exothermic reactions?
reactants to the products
in endothermic reactions the products have more what than reactants?why?
in endothermic reactions the products have more energy than the reactants cuz energys taken in from the surroundings
-first add increasing volumes of sodium (indp v),hydroxide solution to hydrochloric acid,in each experiment we measure the max temp reached.
dep v - max temp reached
control v- volume of hydrochloric acid and conc of hydrochloric acid and sodium hydroxide solution.
-use measuring cylinder to measure 30cm cube of dilute hydrochloric acid,
-transfer acid into plystrene cup,
-stand plystrene cup inside a beaker to stop the cup falling over,
-use thermometer to measure the temp of the acid n record this in a table,
-use measuring cylinder to measur 5cm cube of sodium hydroxide solution
-transfer this to the polystrene cup,fit plastic lid to the cup,
-thermometer through the hole in the lid,bulb of thermometer must be in the solution
-use thermometer to gently stir the solution,this reaction is exothermic=releases energy=temp of solution increases
-look at temp rise on thermometer,when reading on it stops changing we record the highest temp reached,rinse out and dry polystrene cup
-repeat expiremnt using 10cm cube of sodium hydroxide solution
-carry out exp more times,each time inc vol of sodium hydroxide sol by 5cm cube till we reach max of 40cm cube of sodium hydroxide sol
why would you use polystrene cup/why it improves accuracy and why use lid
use plustrene cup - a good/better insulator so it reduces heat loss through the sides and bottom,lids reduce heat loss to the air
What types of reactions are considered exothermic?
Combustion(Burning),some oxidation reactions and neutralization reactions.
whats endothermic
endothermic reactions take IN energy from their surroundings
What effect do endothermic reactions have on the temperature of their surroundings?
They cause a decrease in temperature.
2 uses of exothermic
hand warmers,self heating cans e.g for food or drink
e.g of endothermic
thermal decomposition
for reactions to happen in both endo/exo what do particles have to do
for reactions to happen particles have to collide with eachother
whats activation energy?
the minimum amount of energy that particles must have in order to react is known as the activation energy
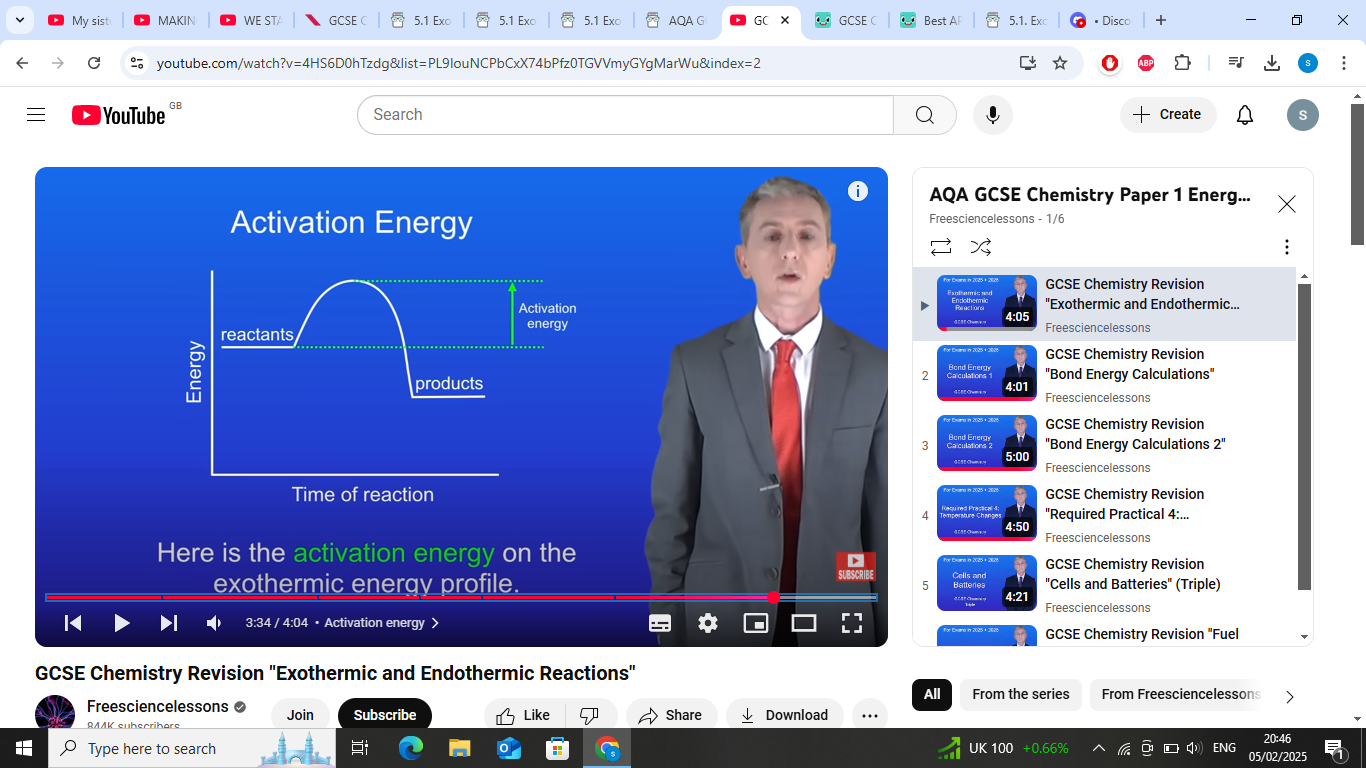
wheres activation energy in graph
reaactants to the peak
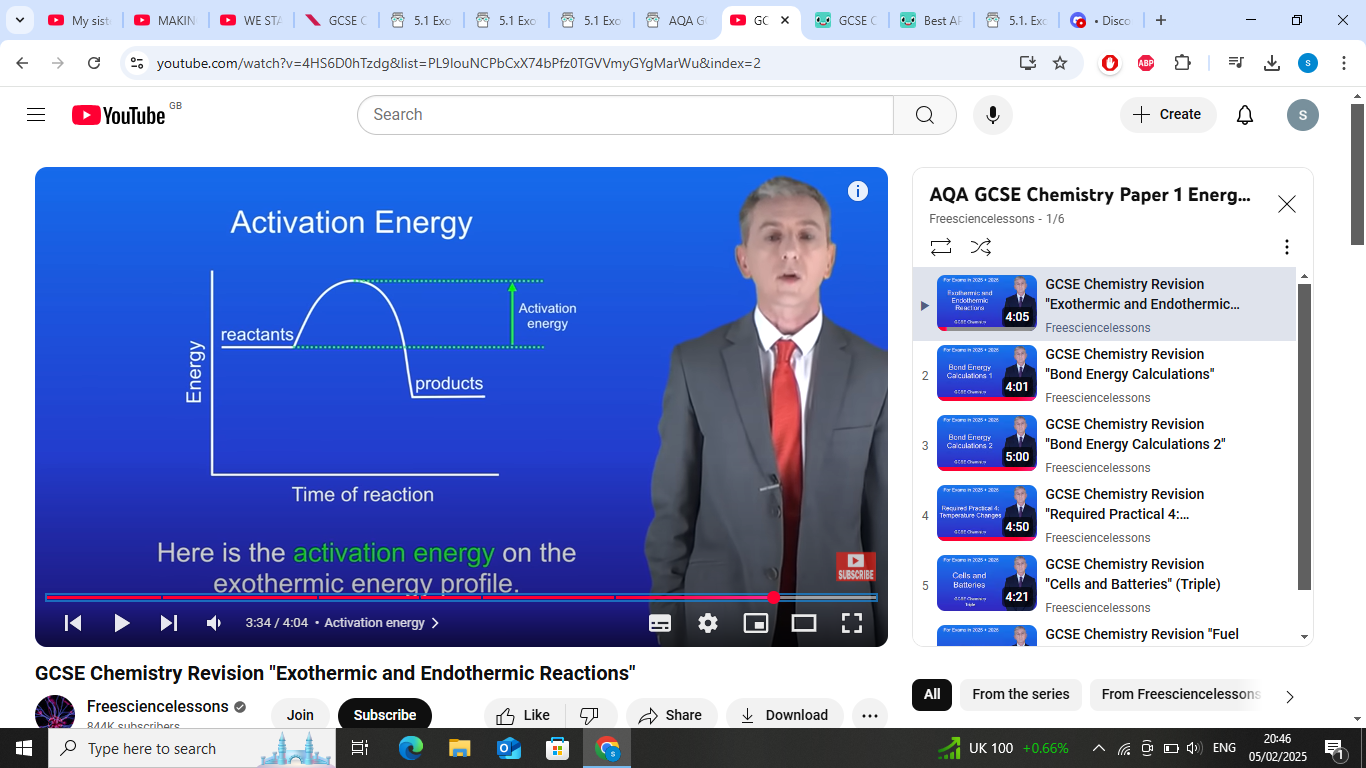
In which types of reactions is activation energy relevant?
Both exothermic and endothermic reactions.