intracellular signalling - transmitting signals within cells
1/23
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
24 Terms
what is a transmembrane receptor and why are they needed
Transmembrane receptor: a cell surface receptor that is either anchored in the membrane or passes through the membrane that binds to extracellular ligands
Proteins, peptides and charged molecules cannot cross the lipid bilayer
Integral membrane proteins transmit the signal into the cell - these receptors span the membrane
Ligands may interact directly with receptors or by binding to co-receptors or accessory molecules on the cell surface
Receptor activation causes a conformational change in the tertiary or quaternary structure that allows the initiation of signalling
what are the 4 ways to transduce a signal
Hydrophobic proteins - membrane associated
Hydrophilic proteins - in the cytosol
Second messengers - cAMP
Ions - Ca2+
what is the purpose of signal amplification
Some stages amplify the signal
Sometimes many signals are needed to activate a pathway
Some signalling molecules activate any pathways causing distribution
Some signals might prevent pathways being activated
what are the 3 ways that signalling molecules are controlled
Post translational modification - phosphorylation
By regulating whether a G protein has bound to GDP or GTP
By provision of activators - Ca2+ and cAMP
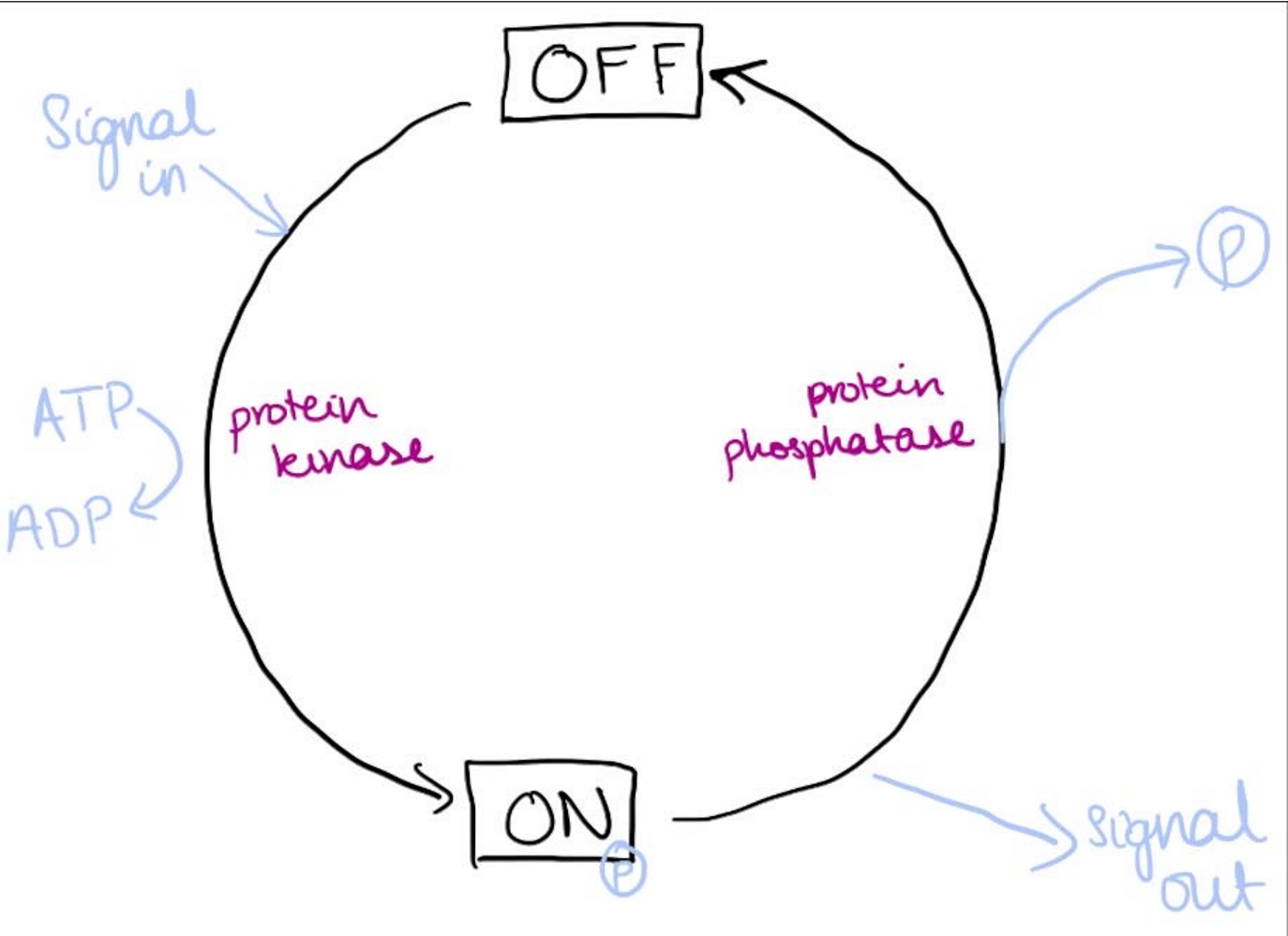
what are the different ways a protein can be phosphorylated and dephosphorylated, also include a description of the cycle
Kinase phosphorylates proteins
Serine/ threonine kinases
Tyrosine kinases
non receptory tyrosine kinases
receptor tyrosine kinases
Phosphatases dephosphorylates proteins
There are some proteins which when phosphorylase’s become inactive
Only 3 amino acids can be phosphorylated: serine, threonine and tyrosine can be activated by these enzymes as they have free hydroxyl groups
what activates the different kinases and what for
Serine/ threonine kinases
Activated by Ca2+/ calmodulin-dependent protein kinases (CaM kinases) phosphorylate transcription factors and myosin during muscle contraction
Tyrosine kinases
Non receptor tyrosine kinases such as Src family kinases for cell proliferation
Receptor tyrosine kinases (RTKs) such as epidermal growth factor receptor
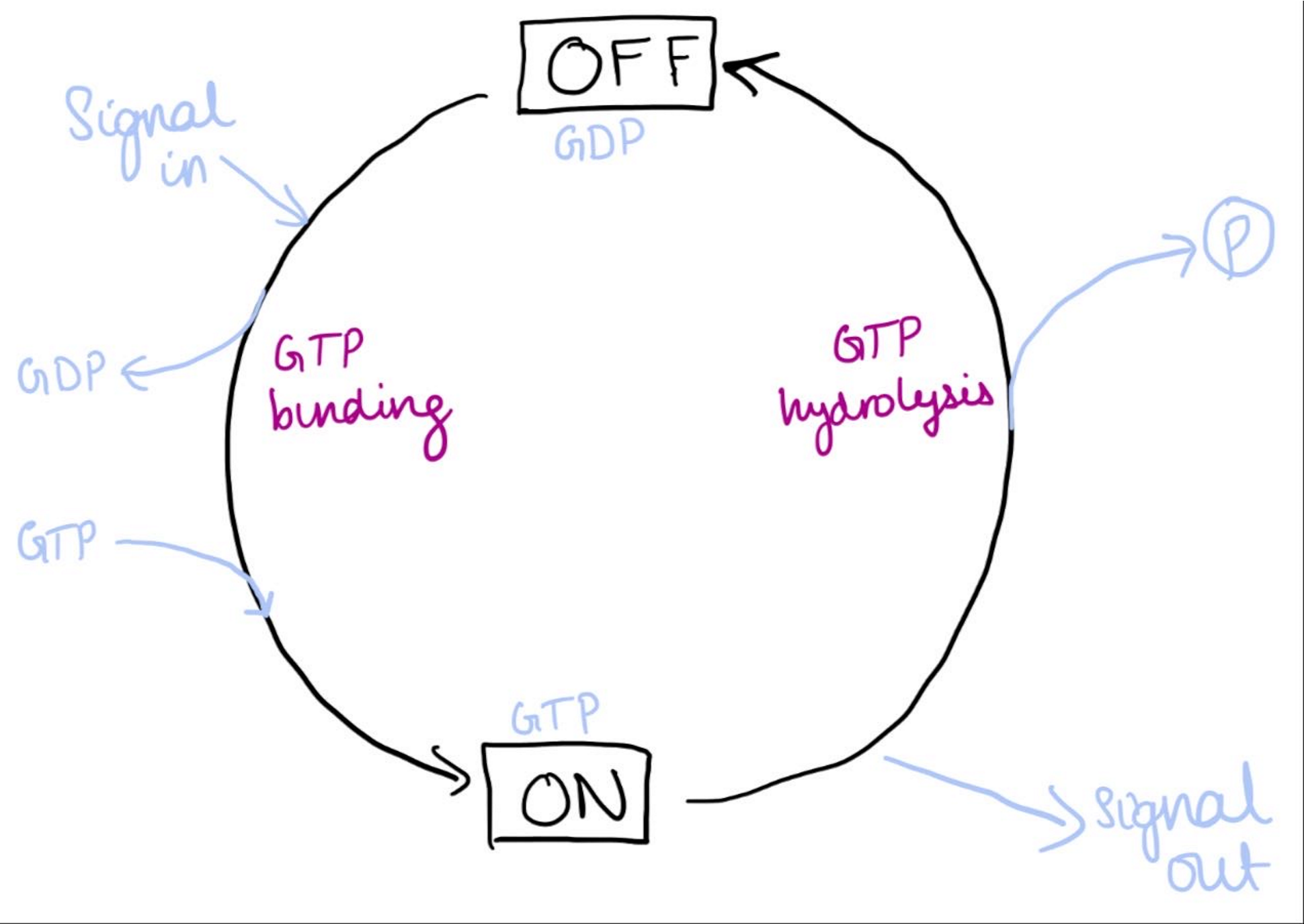
what is the function of GTP binding proteins, when are they used and what affects these proteins
Molecular switch mechanism which are on when binding GTP and off when binding GDP
Used by individual proteins (small GTPases), also heterotrimeric G proteins in GPCR signalling
G protein are always inactive when bound to GDP
Guanine exchange factors (GEFs) promote exchange of GDP for GTP
Hydrolyse GTP-GDP by their intrisin GTPase activity. GTPase-activating proteins (GAPs) speed this up
GTP binding proteins switch mechanism cycle describe
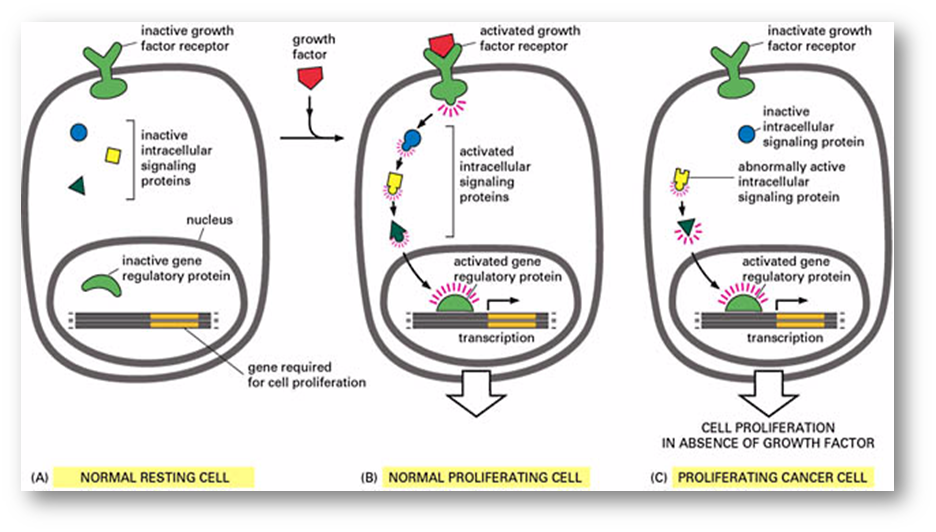
what causes uncontrolled signalling and what is the effect
Ras is a small GTPase
Mutations can cause loss of GTPase activity so there is an abnormally active intracellular signalling protein
Ras mutations found in a large proportion of adenocarcinomas
Yellow signalling protein in diagram is ras
what are the 2 provisional activators of proteins and how do they work
Calcium
Calcium is needed to activate the calmodulin
Calmodulin has to bind to Ca2+ to cause it to change shape and then it folds up and wraps around the kinase which activates it by interacting with it
cAMP
Binding changes the conformation of the target proteins which changes their activity
3 main categories of membrane receptors
Linked to ion channels
Linked to G proteins
Linked to enzymes
function, structure andproperties of ion channels
Transport ions along electrochemical gradient
Specificity of channel is defined by the amino acids lining the channel
Channels are formed of protein subunits
Fast regulated opening/closing mechanism
how are ion channels activated
Change in membrane voltage - voltage gated ion channels: Na+ ion channel
Alpha subunit contains 4 homologous domains forming the pore which opens in response to a change in voltage
4 beta subunits traffic the channel and regulate its kinetic properties
Na+ channel is too small for K+ and it contains negatively charged amino acids to stop Cl-
Alpha subunit has 4 homologous domains which each have 6 transmembrane regions
Region 4 has amino acids with positive R groups which sense the voltage across the membrane causing movement of region 4 opening the channel
Ligand - ligand gated ion channels
Transmembrane proteins consisting of a receptor part and a channel which traverses the membrane
Opens in response to binding of a ligand
Receptors are often classified based on which agonists they bind
Nicotinic acetylcholine receptor
5 subunits: 2 alpha, beta, betagamma and gamma
Transmembrane region M2 of each subunit forms the channel
2 acetylcholine molecules bind the alpha subunits causing movement of the M2 helices opening the channel
When the channel is closed, the leu side chains close the channel, when the M2 helices are made smaller due to twisting of these helices so channel opens
what is the role of ion channels in ion signalling - include how Ca2+ is mediated
Ca2+ regulates secretion, transcription factor activities, skeletal muscle contraction
Effects of Ca2+ are mediated through:
CAM kinases
Calcineurin
Ca2+ is normally kept at low levels in the cytoplasm by ATP dependent pumps
Ca2+ channels are ligand or voltage gated. Activation causes a transient increase in Ca2+
structure of GPCR
GPCRs typically have 7 transmembrane domains
They do not form an ion channel pore
There is a G protein free floating along the inner leaflet of the membrane which consists of 3 subunits
how are GCPRs activated
When a ligand binds to the receptor the affinity for the G protein increases
The receptor and the G protein may sometimes already be in a complex at the membrane
Receptor activation changes the conformation of the internal portion of the receptor releasing GDP
how to GPCRs initiate signalling
GDP attached to the alpha subunit is replaced by GTP
The alpha subunit and the bg Complex dissociate and then each can initiate further signalling
GTP is then hydrolysed to GDP and bg Recombines with an alpha ready to associate with another GCPR
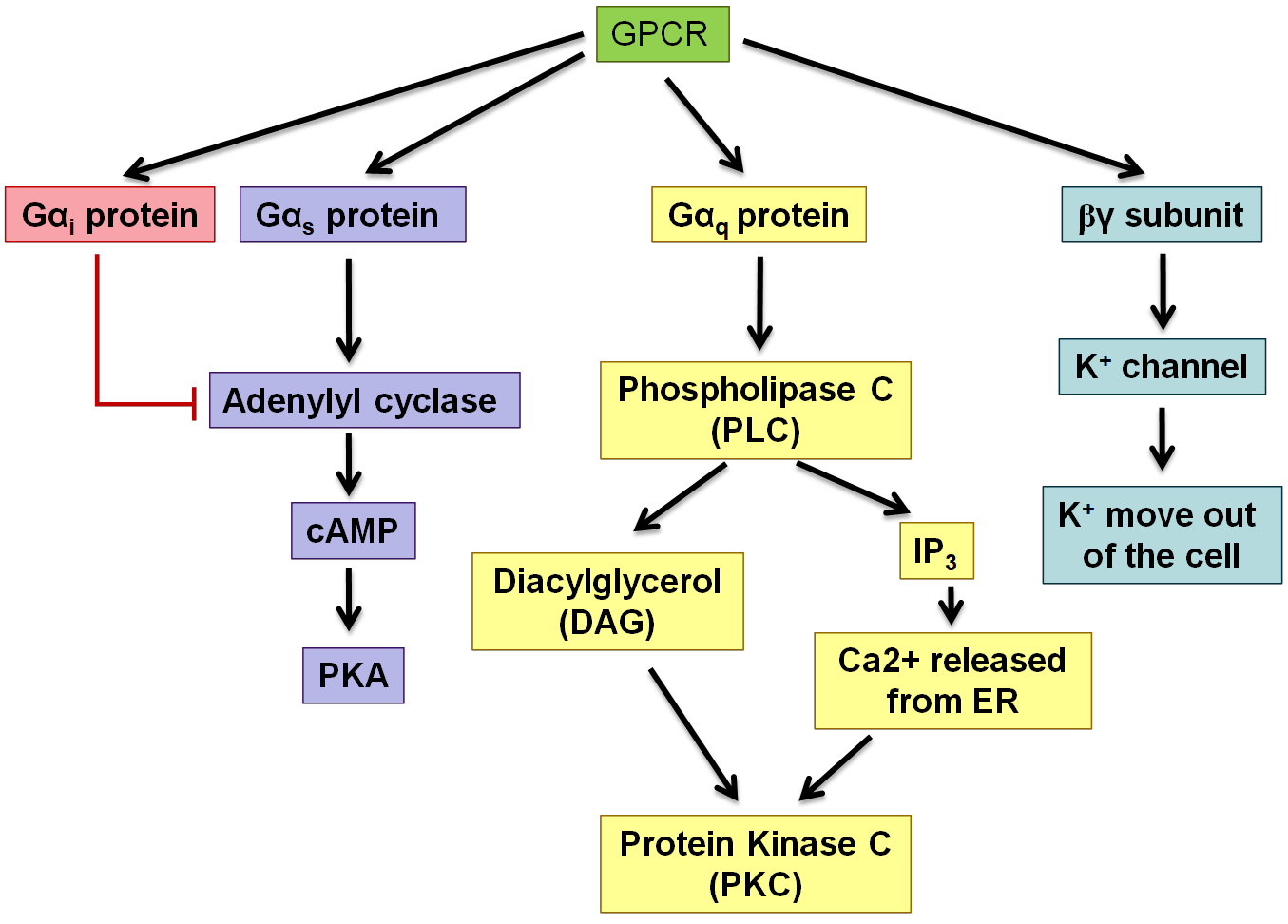
example of GPCR pathway
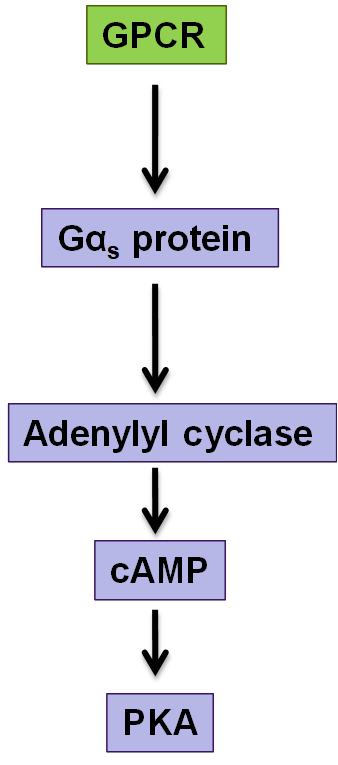
adenylate cyclase and cAMP, beware of which alpha subunit is acitvated for each of the different pathways
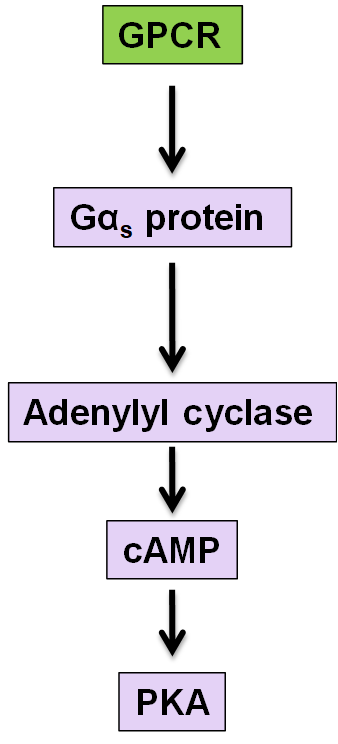
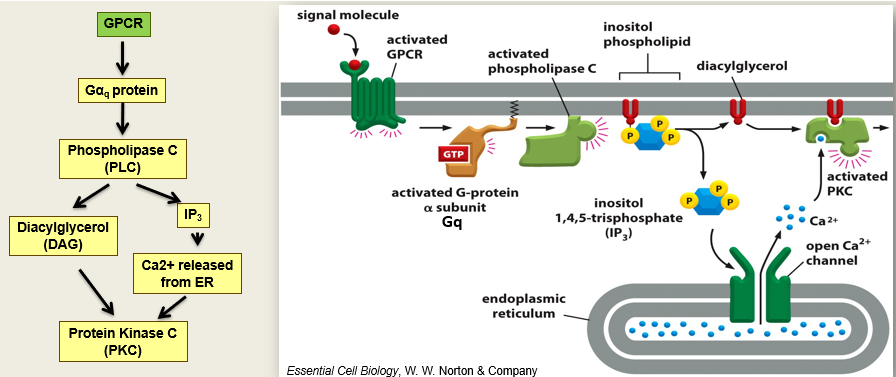
phospholipase C activation
Activates G alphaQ
Activated PLC which cleaves inositol phospholipids in the membrane that releases IP3 which opens calcium channels
Release of calcium leads to secretion which activates PKC
Diacylglycerol also activates PKC
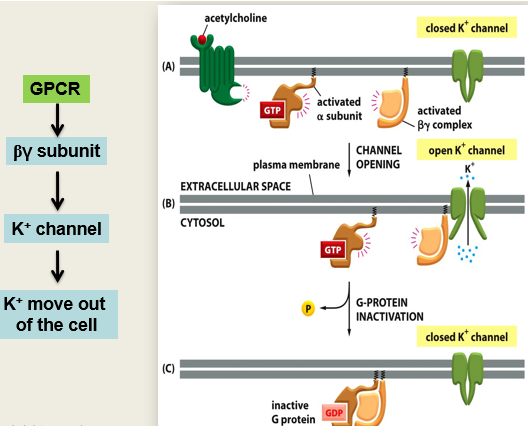
how do GPCRs activate ion channels - use example of slowing down heart rate
Acetylcholine binds to the GPCR (muscarinic M2 receptor in the heart), which activates Gi subunit which inactivates adenylyl cyclase from functioning
the activated bg Unit binds to the K+ channel causing it to open. K+ flows out and the cell becomes hyperpolarised and the heart slows down
Subunit was activated because it was bound to GTP, when this is hydrolysed back to GDP it becomes inactive. When this happens it will come across the bg Subunit on the membrane and they will now recombine and the whole system is turned off again
how does cholera affect signalling pathways
Acute bacterial infection of the intestine
Bacterium Vibrio Cholerae produces cholera toxin which stops this signalling pathway from being able to turn off
Inhibits GTPase activity of the subunit G alphaS (stimulates adenylyl cyclase)
Prolonged signalling so lots of cAMP which keeps activating the CL- transporter so causes water and Cl- to move out of the cells lining the intestine
Results in diarrhoea, severe dehydration and death
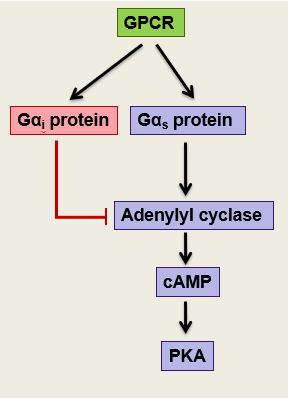
how does whooping cough affect signalling pathways
Bacterial infection of the lungs
Bordetella pertussis bacterium releases an active adenylyl cyclase domain so activates the signalling pathways by producing cAMP
Pertussis toxin renders G alphaI inactive (G alphaI inhibits adenylyl cyclase)
Prevents natural way of turning off adenylyl cyclase so it cant bind to receptor so lots of stimulation
Modifies G alphaI preventing association with GPCRs
Prolonged signal stimulates coughing
how do receptors linked to enzymes work
Either have enzymatic ability built into receptor (so are tyrosine kinase receptor) or first step in signalling will be a kinase (serine, threonine, tyrosine)
Usually have a single membrane spanning domain and come together
Response usually requires receptor dimerisation (2 parts of receptor coming together)
Homo dimers - 2 of same protein coming together to form receptor
Heterodimers - 2 slightly different proteins
Cytoplasmic enzymes that induce signalling are normally protein tyrosine kinases
Got kinase activity built into the intracellular domain
Close proximity allows the kinase activity to get activated and they will phosphorylate each other and add lots of phosphate groups at different sites which will then allow first steps in signal transduction cascade to bind as it is now right shape and charge for signalling molecules to dock
Ligand binding activated enzyme activity within the cytoplasmic domain
Tyrosine residues in the intracellular domains are auto-phosphorylated in response to the signal