GAS LAWS AND KINETIC MOLECULAR THEORY OF GASES | 3.4
0.0(0)
Card Sorting
1/14
There's no tags or description
Looks like no tags are added yet.
Last updated 2:44 PM on 10/4/25
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
15 Terms
1
New cards
> Pressure
> Volume
> Moles (Amount of Gas (n))
> Temperature
Macroscopic Properties of Gases
2
New cards
Pressure
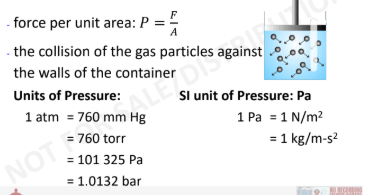
3
New cards
Volume
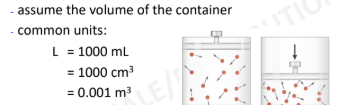
4
New cards
Amount of Gas (n) moles
number of particles
- unit: mol
5
New cards
Temperature, T
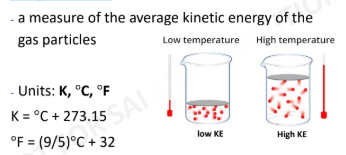
6
New cards
Boyle’s Law:
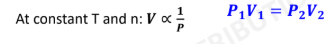
7
New cards
Charle’s Law
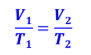
8
New cards
Avogadro’s Law:
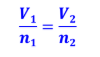
9
New cards
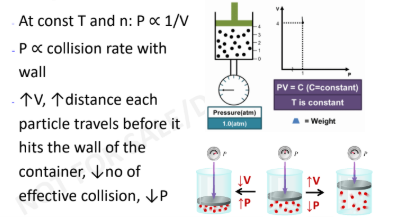
Boyles Law
10
New cards
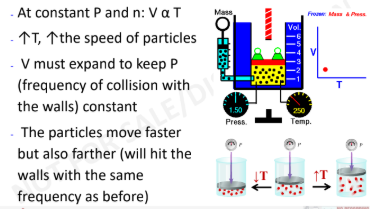
Charles’ Law and KMT
11
New cards
Avogadro’s Law and KMT
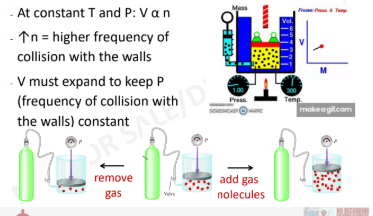
12
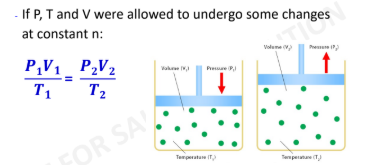
New cards
Combined gas law
13
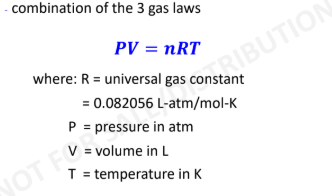
New cards
Ideal Gas Law
14
New cards
Ideal Gas
> gas have neglible volume
>elastic collisions
> no interaction gas particles (IMFA insiginificant)
15
New cards
Real Gas
> Gases have volume
> energy is lost in collisions
> IMFA significant