Topic 5- Transition metals,alloys and corrison
1/17
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
18 Terms
what type of metal are most metals
transition metals
what are the properties of transition metals
High melting point
High density
Formation of coloured compounds
Catalytic activity of metals and their compounds
As exemplified by iron
what does the oxidation of metals result in
corrosion
what is corrosion
Breaking down of metals due to chemical reactions , usually with oxygen
what is rusting
rusting is specifically the corrosion of iron (only metal that rusts, others corrode) in the presence of oxygen and water
what is rust
hydrated iron (III)
What is the formation of rust
iron + water + oxygen → hydrated iron (III) oxide
Explain how rusting of iron can be prevented by:
a exclusion of oxygen
Using a physical barrier:
Coating the iron with oil or paint
Both provide physical barrier between the iron, oxygen and water
Prevents iron from reacting with oxygen
Explain how rusting of iron can be prevented by:
b exclusion of water
Using a dessicant
A substance that absorbs the water vapour so it keeps the iron dry
Explain how rusting of iron can be prevented by:
c sacrificial protection
Involves attaching big blocks of magnesium or zinc to the iron
Because magnesium is more reactive than iron it will corrode first leaving the iron intact
Magnesium blocks replaced because they have been corroded immediately
Mg → Mg2+ + 2e-
how can electroplating be used to improve the appearance and / or the resistance to corrosion of metal object?
electroplating coats the surface of one metals with a thin layer of another. to improve appearance, expensive metals such as silver and gold can be used. to improve a metal's ability to resist corrosion, chromium is often used because it stops air and water reaching the steel below, preventing it from rusting. electronic equipment and expensive hi-fi cable may use gold-plated connectors because gold is the third-best electrical conductor, and does not tarnish.
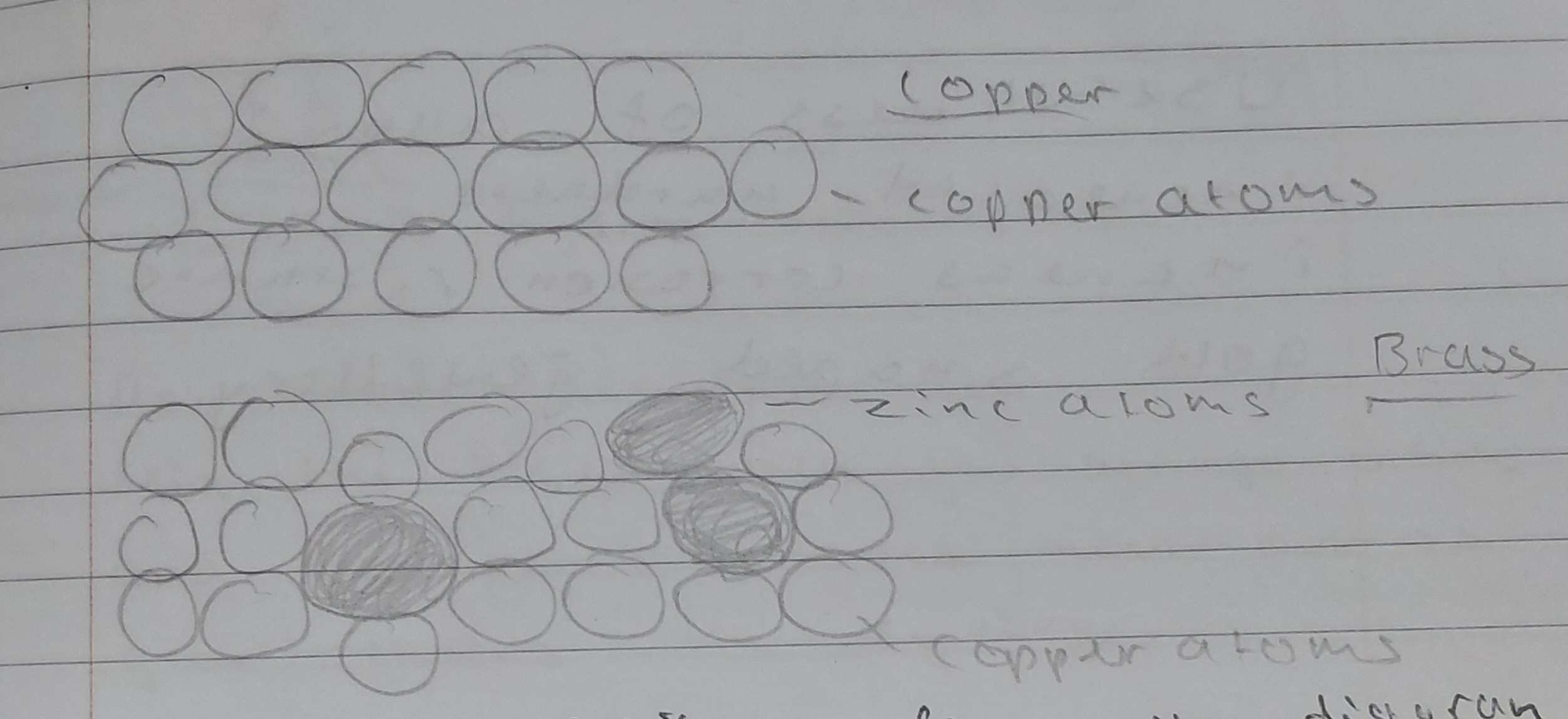
Explain, using models, why converting pure metals into alloys
often increases the strength of the product
an alloy is formed when a small amount of another metal is added to an otherwise pure metal
It alters the regular arrangement of the atoms
So the layers no longer slide easily past one another
Making the alloy harder than the pure metal
why is iron alloyed with other metals to produce alloy steels?
pure iron is too soft for everyday use, but wrought iron is stronger, even though it only contains a small amount of other elements. alloy steels are even stronger than wrought iron.
what are the properties of aluminium, and what is it used for?
aluminium resists corrosion, but it does not conduct electricity as well as copper. however, it is stronger, cheaper, and less dense, so it is used for overhead electrical cables.
what are the properties of gold, and what is it used for?
golds resists corrosion, and is also malleable, ductile, and a very good conductor of electricity. however, it is very expensive, so instead of using it for most electrical wiring, it is used in tiny amounts to connect microprocessors and memory chips. jewellery
what are the properties of copper, and what is it used for?
copper resists corrosion. it is also malleable, ductile, and a very good conductor of electricity. it is not as good as gold, but is thousands of times cheaper, so it is chosen for most electrical wiring.
what are the properties of magnalium, and what is it used for?
magnalium is less dense and almost four times stronger than aluminium alone, and although it is denser than magnesium, it is twice as strong, and has better resistance to corrosion. these properties allow the manufacture of strong but lightweight metal parts used for aircraft parts and scientific instruments.
what are the properties of brass, and what is it used for?
brass is resistant to corrosion, and I stronger than copper, although it is not as good a conductor of electricity. this makes it more suitable than copper alone for making electrical plug pins.