Topic 1B - Key concepts in biology, ENZYMES
1/48
Earn XP
Description and Tags
2 Core Practical's: 1)Investigate the effect of pH on enzyme activity (1.10) 2)Investigate the use of chemical reagents to identify starch, reducing sugars, proteins and fats (1.13)
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
49 Terms
1.7 what are enzymes?
-proteins which are biological catalysts to speed up the rate of reactions without being used up.
-they allow metabolic reactions to occur at a rate that can sustain life
1.7 how is an enzyme-substrate complex made?
when the substrate moves into the enzyme’s active
1.7 what are the steps of an enzyme catalysed reaction?
enzymes and substrates randomly move around in solution
When an enzymes and its complementary substrate randomly collide, an enzymes substrate complex forms and the reaction occurs
a product (or products) forms (form the substrate) and is then released form the active site. The enzymes is unchanged and will then go on to catalyse further reactions.
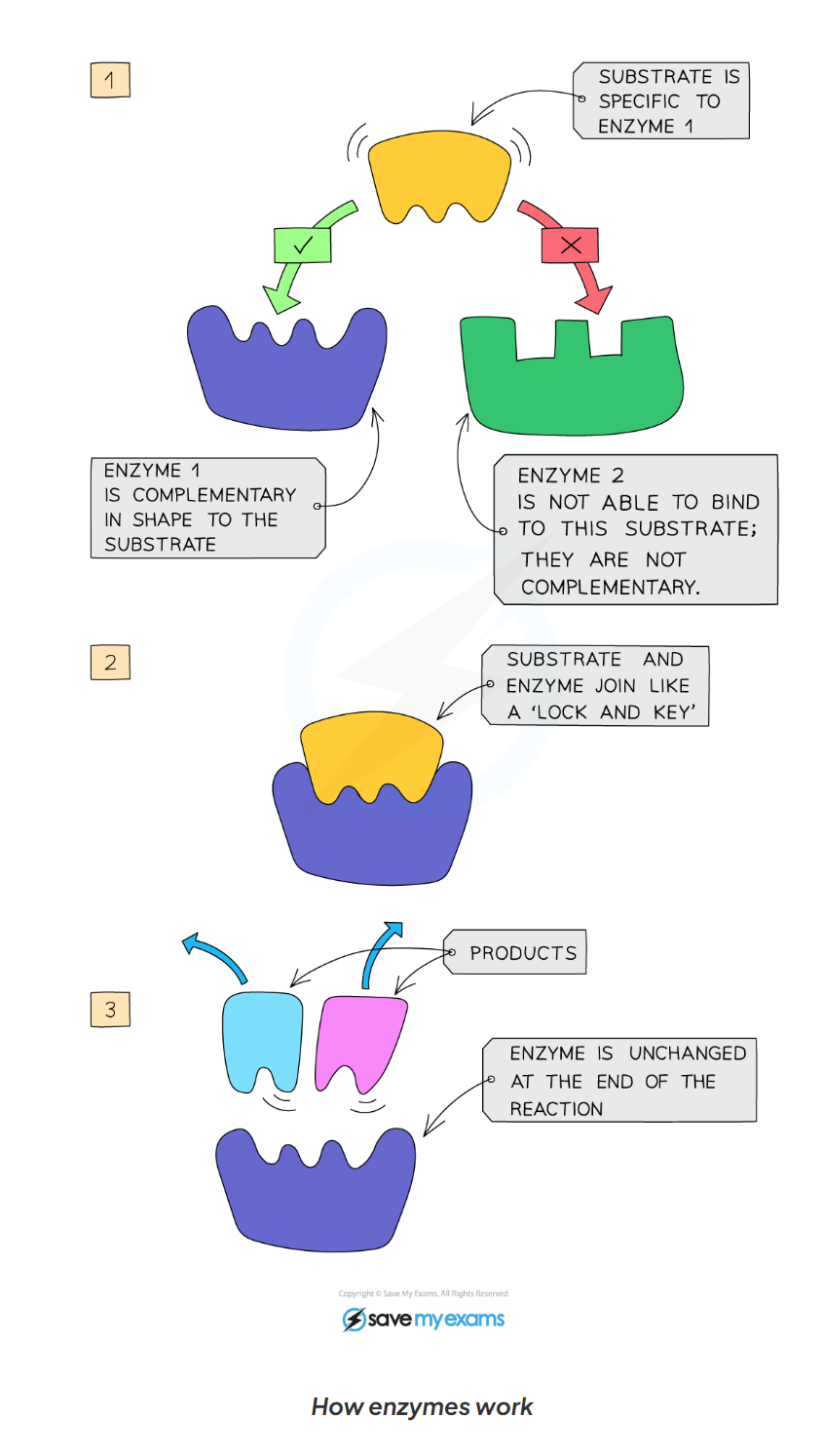
1.7 specificity of enzymes
active site has a specific shape which is complementary to its substrate
1.8 denaturation of enzymes
-if the bonds that hold the enzyme together is disrupted or broken the active site will lose its shape - known as being denatured
-denaturation cannot be reversed
-can occur due to high temps or extreme ph’s
1.8 what happens when an enzymes is denatured?
the substrate cannot fit into the denatured active site
1.9 What factors affect enzyme activity?
-temperature, pH, substrate concentration and enzymes concentration
1.9 the affect of temperature of enzymes activity
enzymes work fastest at their optimum temperature ( in humans this is 37 degrees C)
-Heating to high temperatures (beyond the optimum) will break the bonds that hold the enzyme together and the active site will lose its shape.
-As temperature increases (towards the optimum) the activity of enzymes increases
This is because the molecules have more kinetic energy, move faster and have more successful collisions with the substrate molecules. This leads to a faster rate of reaction
-This means that low temperatures do not denature enzymes, they just make them work more slowly due to a lack of kinetic energy
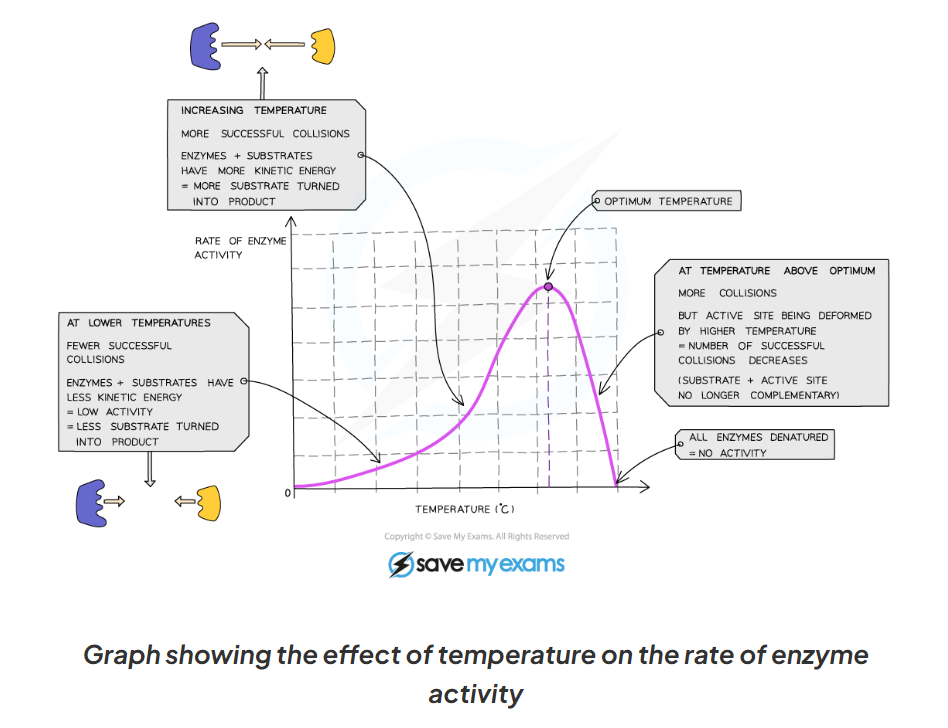
1.9 Explain what is happening at each point of this diagram showing the affect of temp on enzymes activity
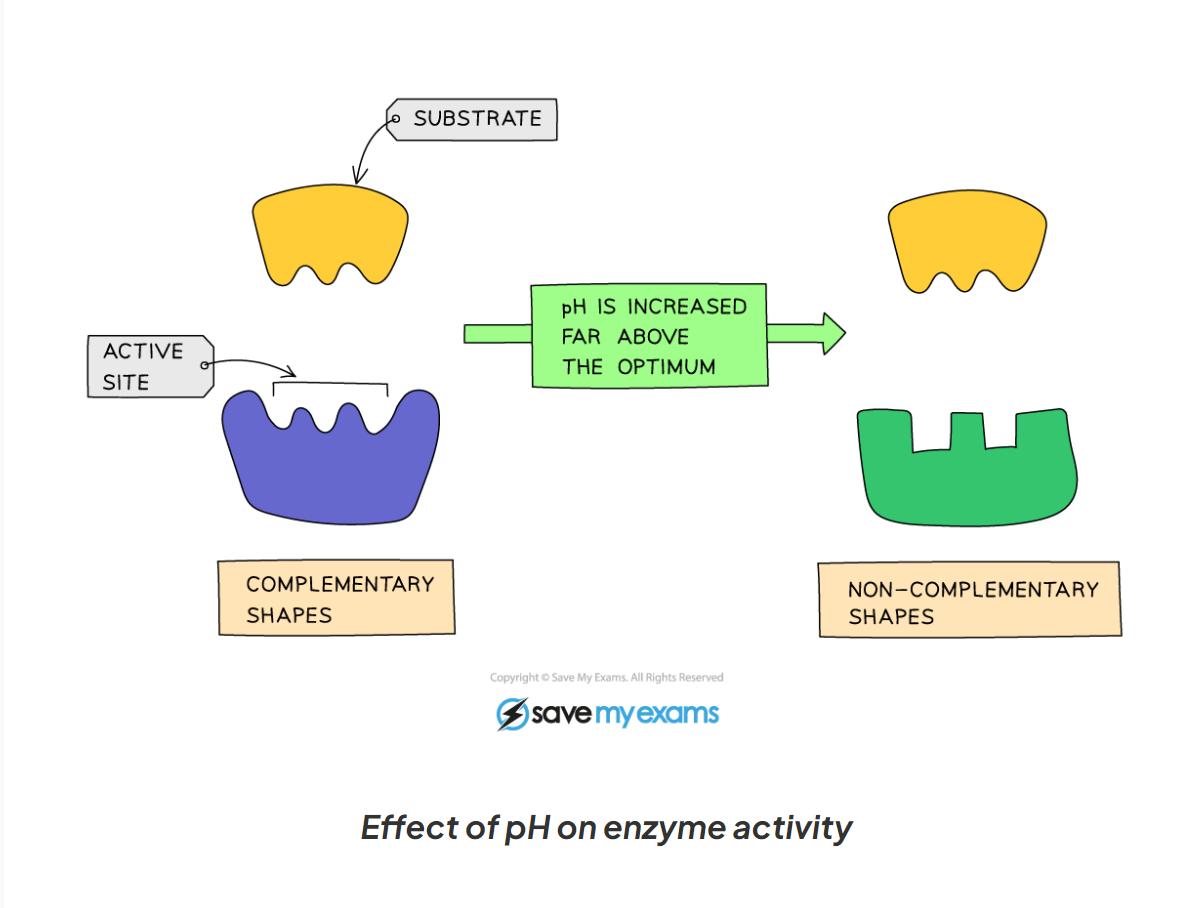
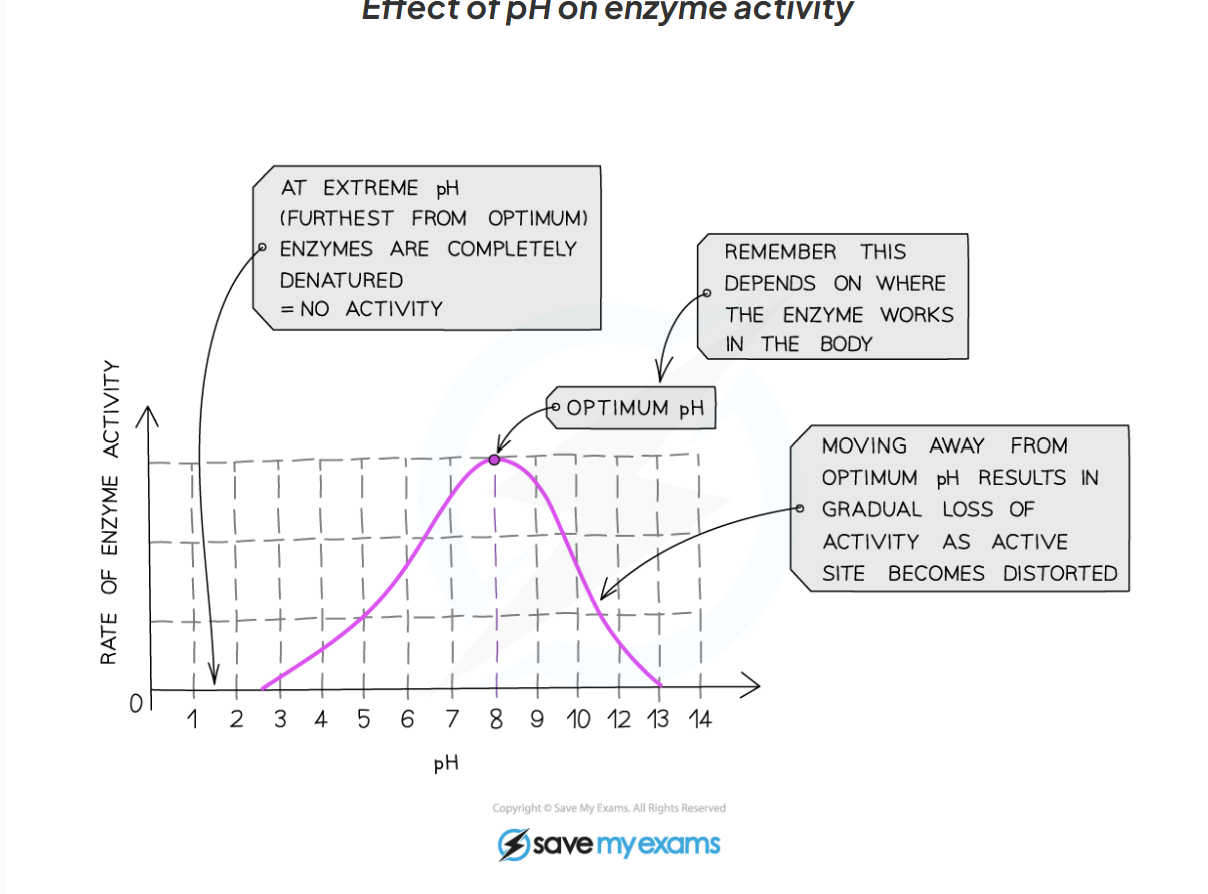
1.9 How does pH affect the activity of enzymes?
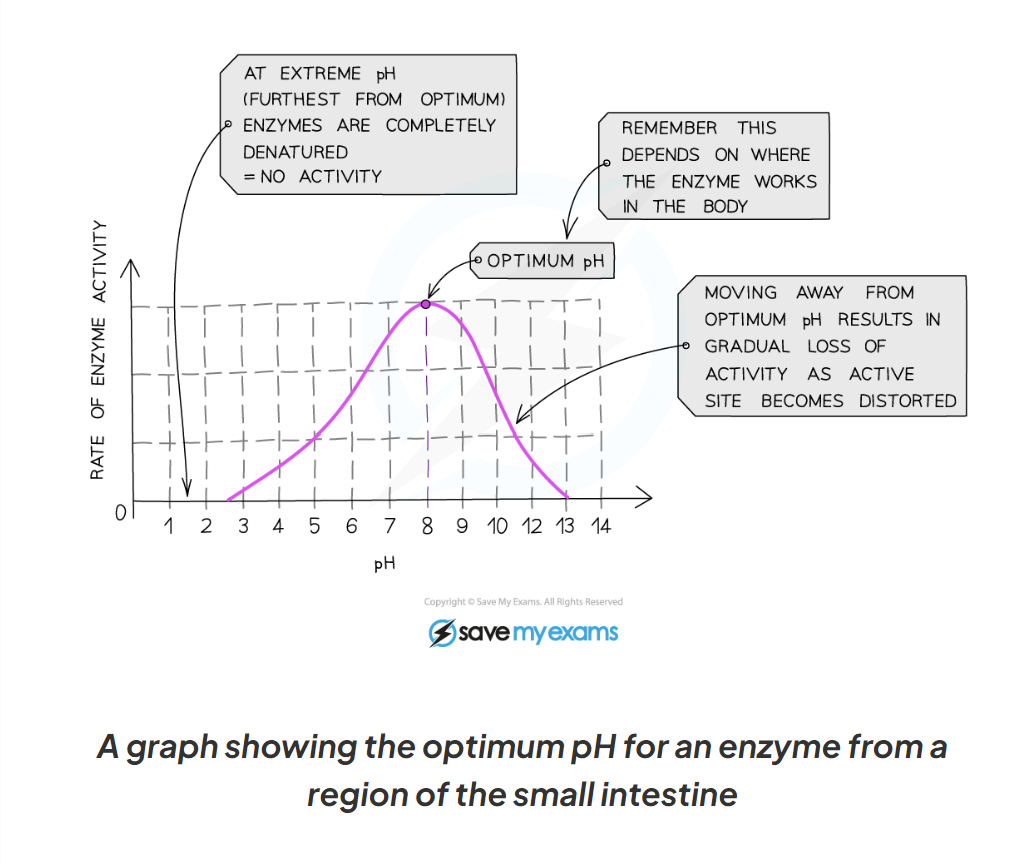
-The optimum pH for most human enzymes is pH 7
Some enzymes that are produced in acidic conditions, such as the stomach, have a lower optimum pH (pH 2)
Some that are produced in alkaline conditions, such as the duodenum, have a higher optimum pH (pH 8 or 9)
-If the pH is far too above or far too below the optimum, the bonds that hold the amino acid chain together to make up the protein an be broken
-this will change the shape of the active site, so the substrate can no longer fit, reducing the rate of activity
-moving too far away form the optimum can cause the enzymes to denature and the reaction is it catalysing will stop.
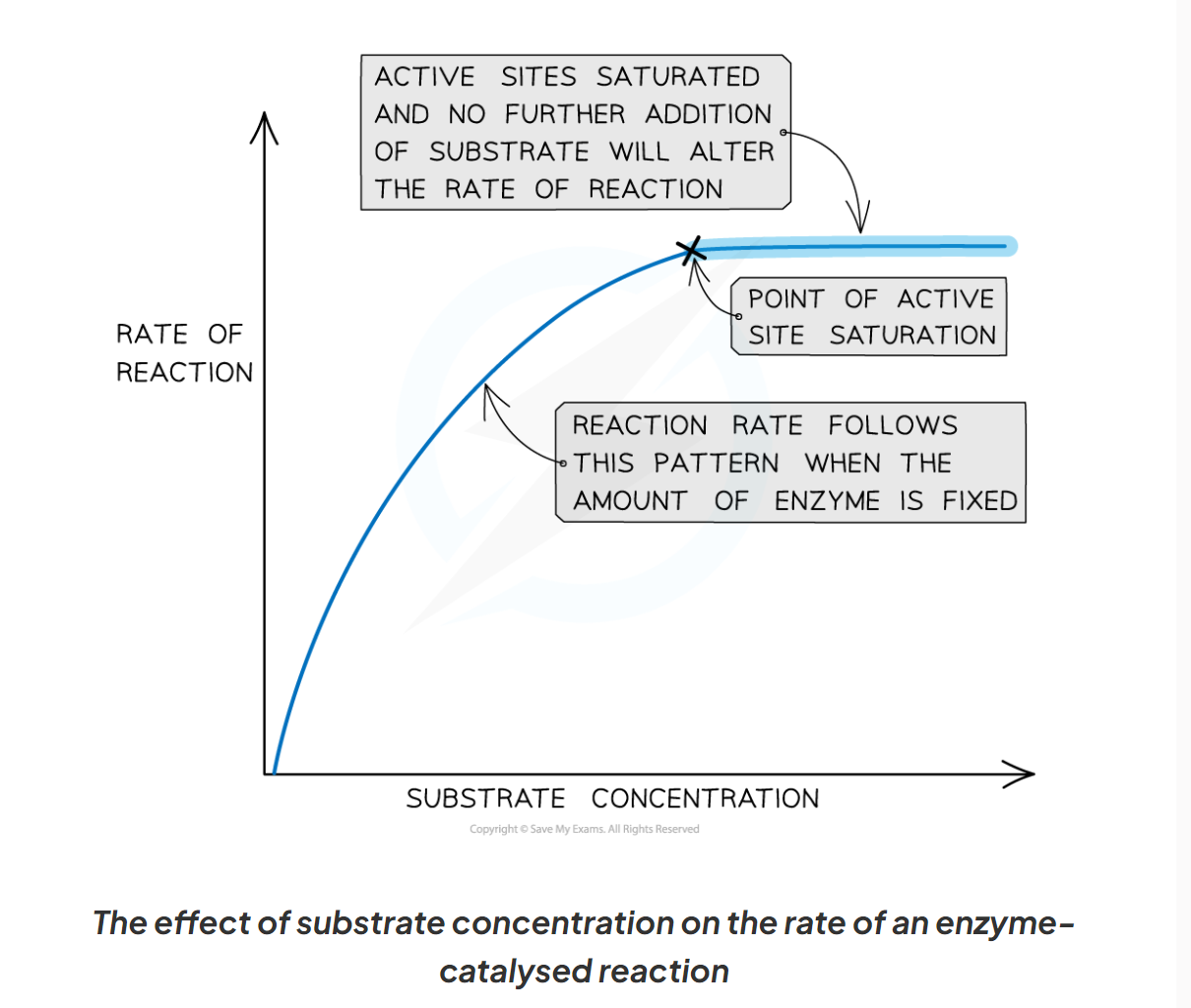
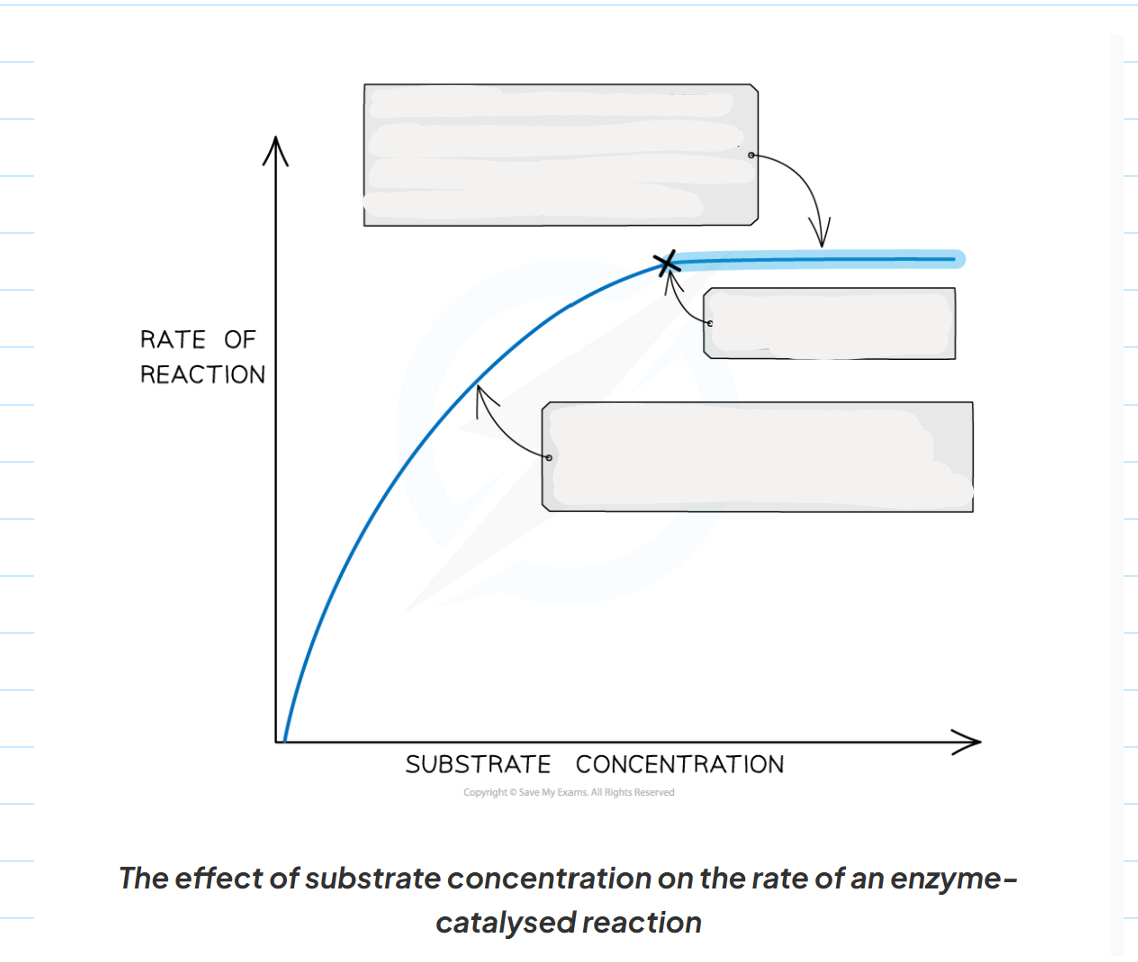
1.9 How does substrate concentration affect enzyme activity?
-the greater the substrate concentration the greater the enzymes activity and the higher the rate of reaction.
-as the number of substrate molecules increases, the likelihood of enzymes-substrate complex formation increases.
-If the enzymes concentration remains fixed but the amount of substrate increased past a certain point, all available active sites eventually become saturated and any further increased in substrate concentration will not increase the rate of reaction.
-when the active site of the enzymes are all full, any substrate molecule that are added haven o where to bind in order to for an enzymes-substrate complex.
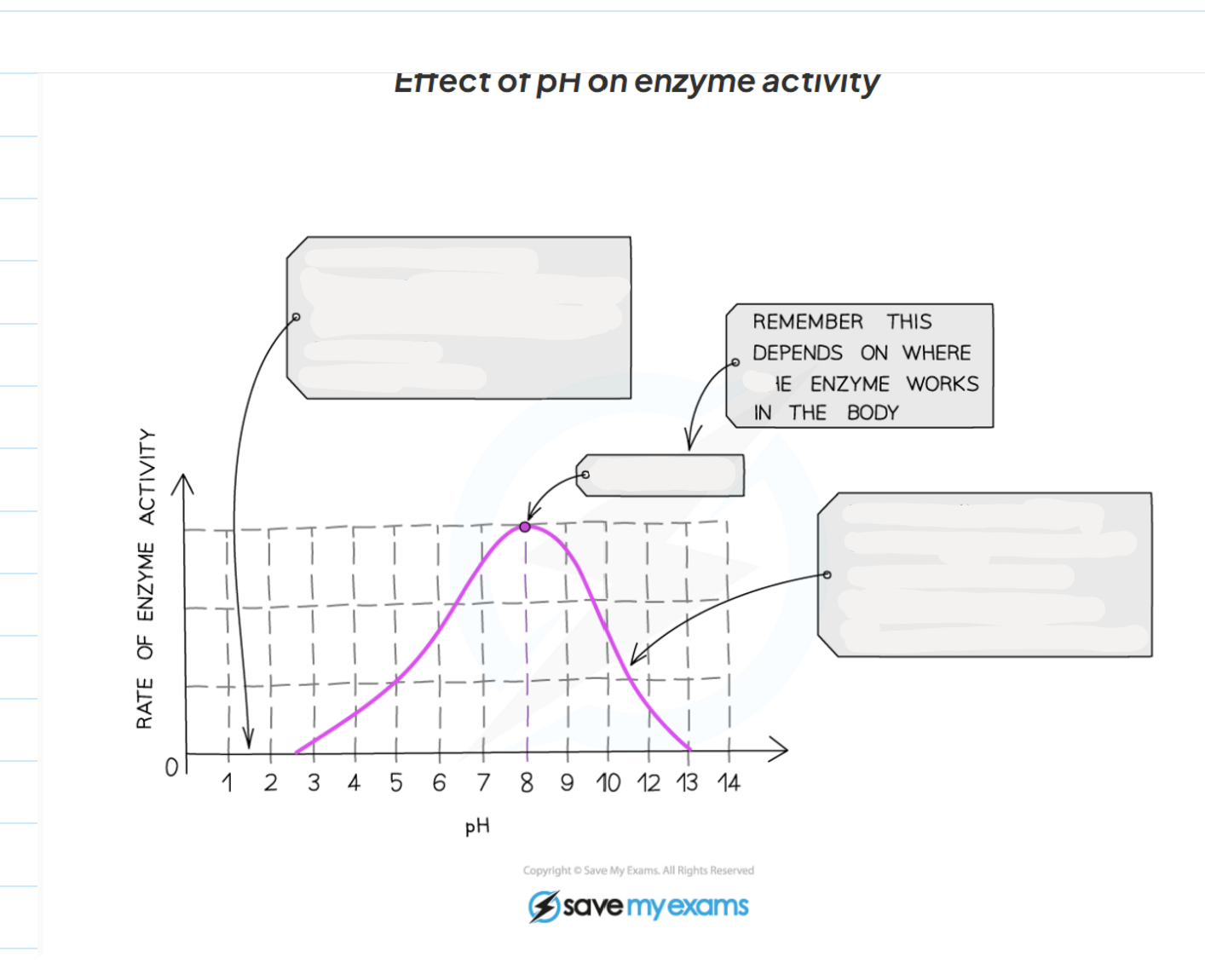
1.9 Label this diagram of the affect of pH on enzyme activity
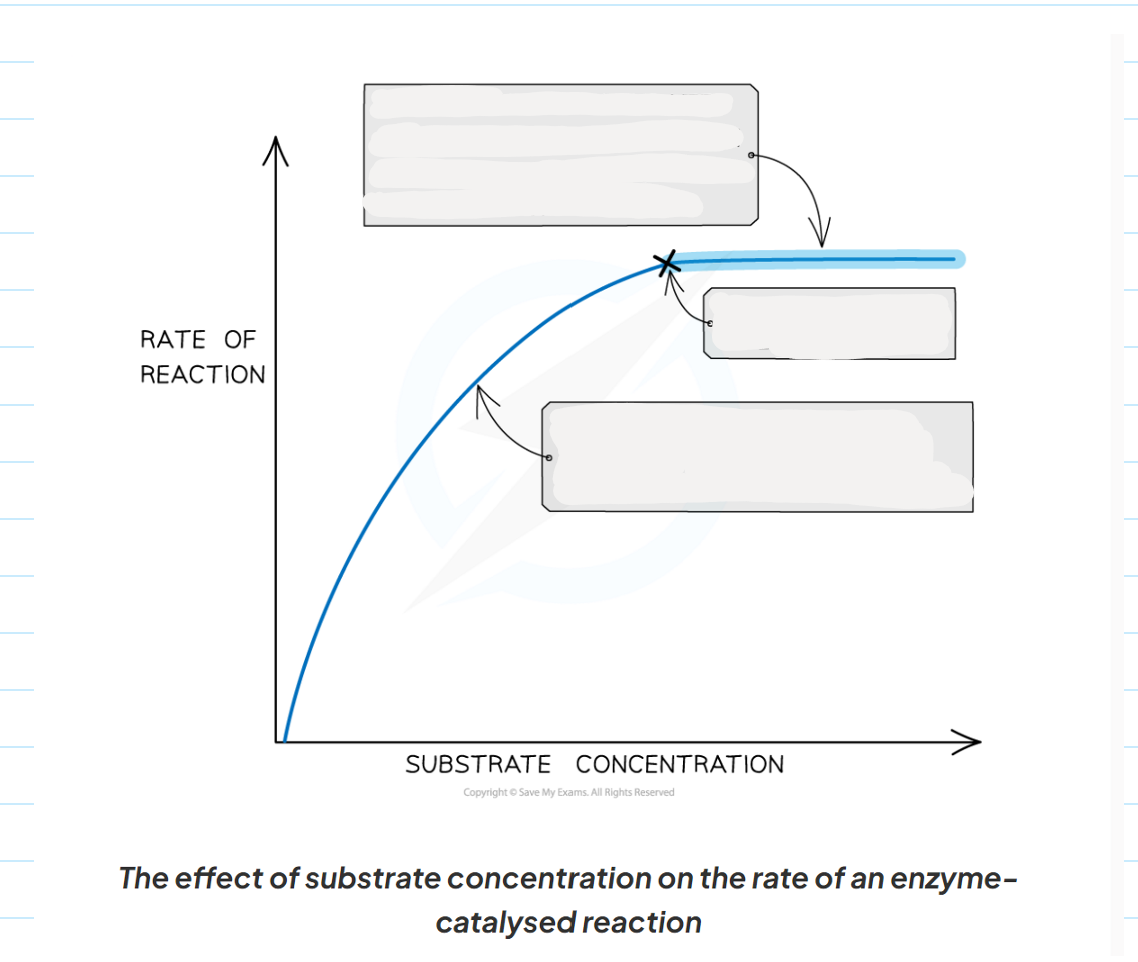
1.9 Describe what is happening at each point of this graph showing the affect of substrate concentration on enzymes activity
1.10 what apparatus are needed for the core practical to investigate the affect of pH on enzymes. (10 things)
spotting tile, measuring cylinder, test tube, syringe, pipette, stopwatch, buffer solutions, iodine, starch solution, amylase solution
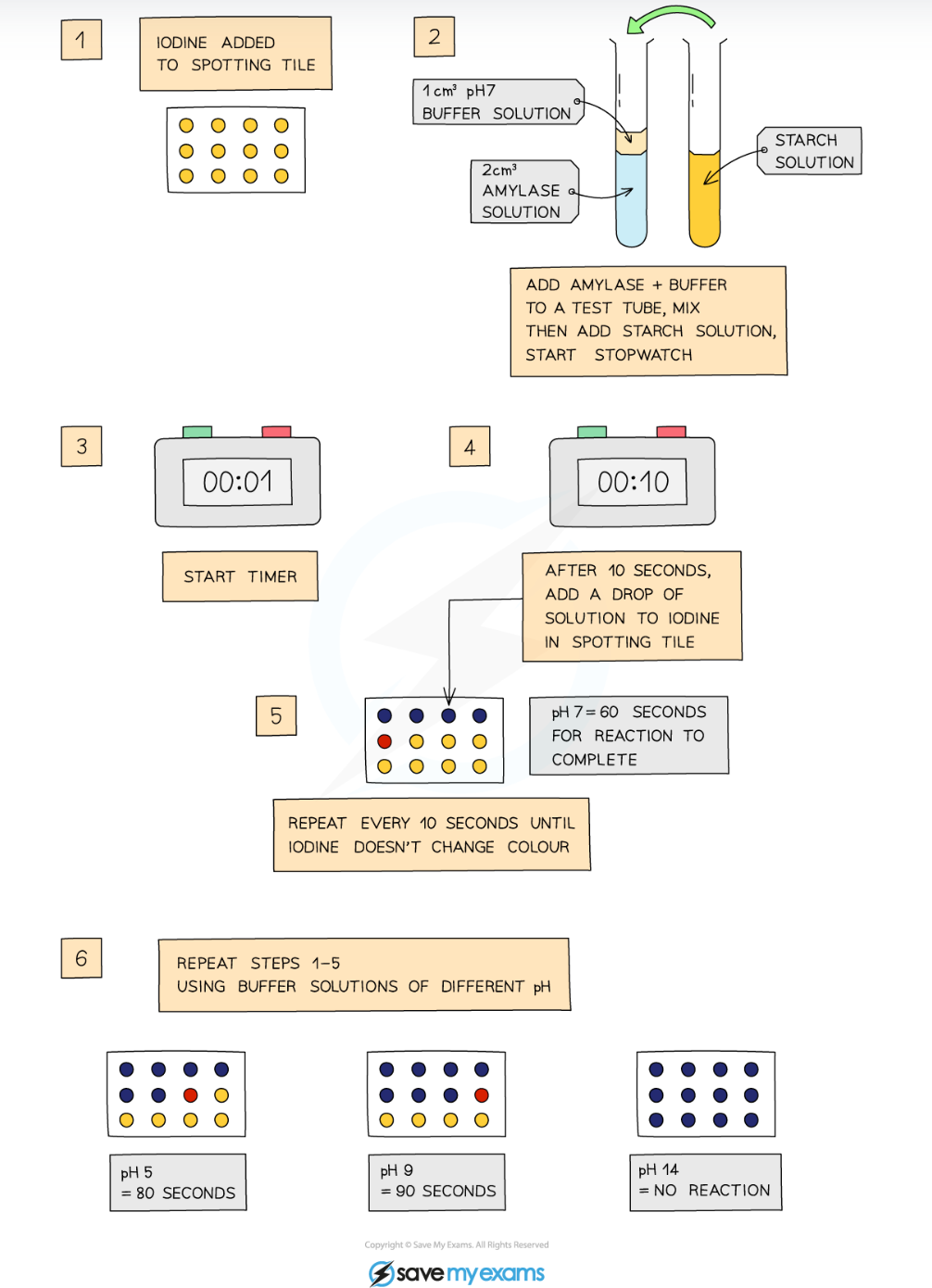
1.10 what is the method to investigate the affect of pH on enzymes
Add a drop of iodine to each of the wells of a spotting tile
Use a syringe to place 2 cm3 of amylase into a test tube
Add 1cm3 of buffer solution (at pH 2) to the test tube using a syringe
Use another test tube to add 2 cm3 of starch solution to the amylase and buffer solution, start the stopwatch whilst mixing using a pipette
Every 10 seconds, transfer a droplet of the solution to a new well of iodine solution (which should turn blue-black)
Repeat this transfer process every 10 seconds until the iodine solution stops turning blue-black (this means the amylase has broken down all the starch)
Record the time taken for the reaction to be completed
Repeat the investigation with buffers at different pH values (ranging from pH 3.0 to pH 7.0)
1.10 Diagram to show the method for the affect of
pH on enzymes
1.10 what are the results and analysis for this experiment investigating the affect of pH on enzymes
This investigation shows:
At the optimum pH, the iodine stopped turning blue-black and remained orange-brown within the shortest amount of time
This is because the enzyme is working at its fastest rate and has digested all the starch
At higher or lower pH's (above or below the optimum) the iodine took a longer time to stop turning blue-black or continued to turn blue-black for the entire investigation
This is because on either side of the optimum pH, the enzymes are starting to become denatured and as a result are unable to bind with the starch or break it down
1.10 What are amylase?
Amylase is an enzyme which breaks down starch
When the iodine solution remains orange-brown, all the starch has been digested
1.10 what should be done to ensure the experiment is fair?
-the starch and amylase solution that need to be used should be place in a water bath at optimum temp before being used.
1.10 what could be used to measure the progress of a reaction more accurately
-a colorimeter can be used to measure the progress of the reaction more accurately by measuring the absorbance / transmission of light through the coloured solution
a control of iodine solution would be and for comparison
1.10 here is a graph showing the optimum pH for an enzymes from a region of the small intestine
1.11 What is the equation to perform a rate calculation? And explain the components in the equation
rate = change / time
change refers to the change in the substance being measured.
-this could be the volume of substance used up in the reaction or the volume of product formed
-time refers to the time taken for the change to occur.
rate = amount of substance used or product formed / time
1.11 Amylase catalyses the breakdown of starch into maltose. 15 grams of starch were added to a solution containing amylase. It took 2 hours for all the starch to be broken down. Calculate the rate of reaction
15g / 2hours = 7.5g/h
1.12 What is the purpose of digestion?
to break down large, insoluble molecules into smaller, soluble molecules that can be absorbed into the blood stream
1.12 How is food digested?
Mechanically: by chewing, churning and emulsification in order to break down larger pieces of food into smaller pieces.
This increases the surface area for enzymes to work on
Chemically: bonds holding large molecules together are broken to make smaller molecules
1.12 What controls chemical digestion?
Enzymes that are produced in different areas of the digestive system
1.12 What are the three main types of digestive enzymes?
Carbohydrase, protease, lipase
1.12 What are carbohydrase and their role in the break down of carbs?
enzymes that break down carbohydrates into simple sugars such as glucose
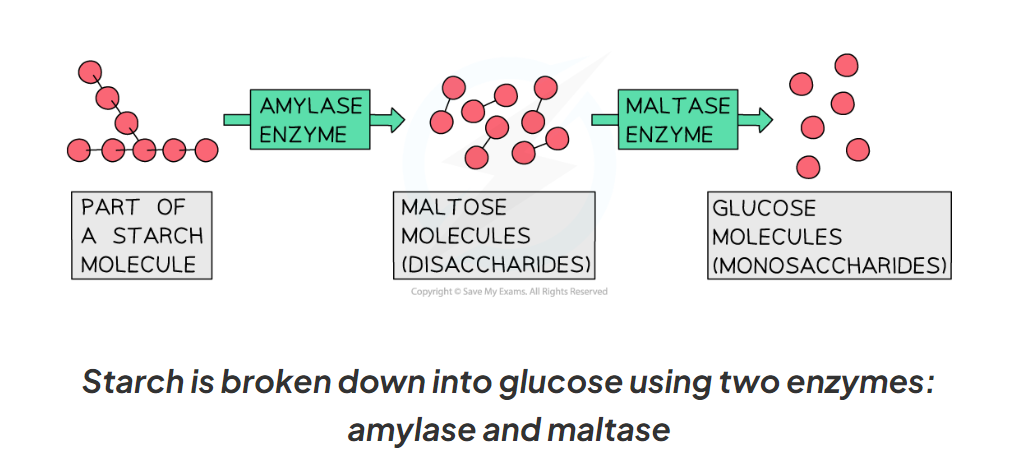
Amylase is a carbohydrase that is made in the salivary glands, the pancreas and small intestine.
Amylase breaks down starch into maltose
Maltase then breaks down maltose into glucose
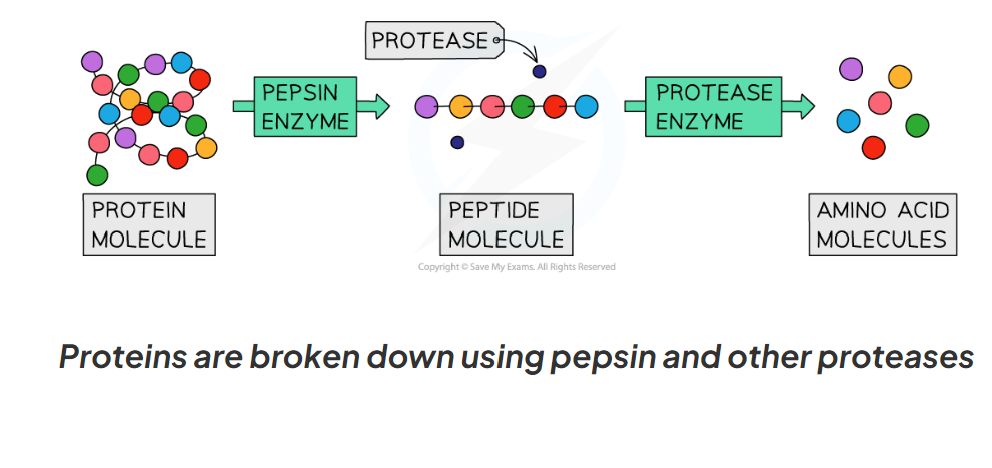
1.12 What are proteases and their role in the break down of protein?
Proteases are a group of enzymes that break down protein into amino acids
Pepsin is an enzymes made in the stomach that breaks down proteins into smaller polypeptide chains
Proteases made in the pancreas and small intestine break the polypeptides into amino acids
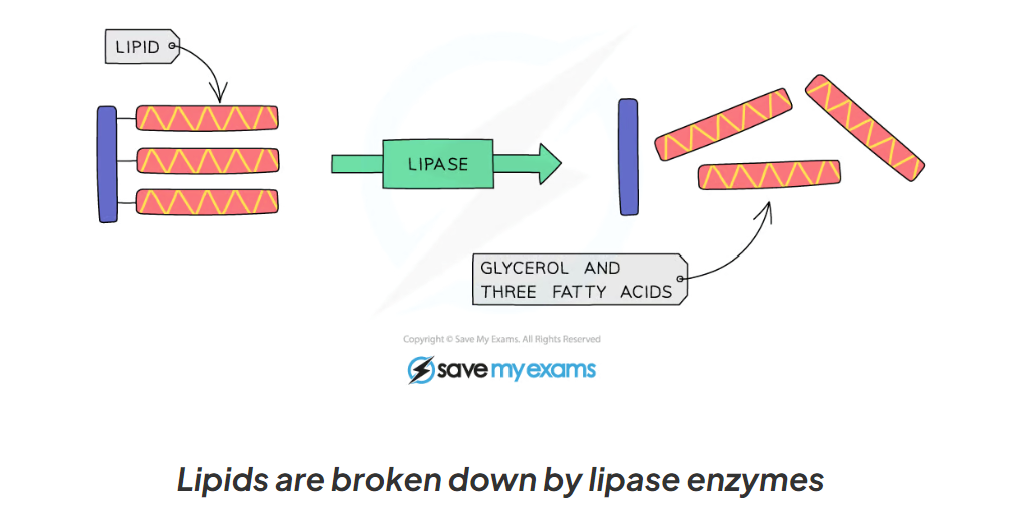
1.12 What are lipase and their role in the break down of lipids?
Lipase are enzymes that break down lipids (fats) to glycerol and fatty acids
Lipase enzymes are produced in the pancreas and secreted into the small intestine
1.12 How does synthesis of protein, carbs and lipid happen?
enzymes are required by organisms to synthesis carbohydrates, proteins and lipids
1.12 How are carbohydrates synthesised?
by joining simples sugars together
for example, glycogen synthase is an enzymes that joins together many chains of glucose molecules to form glycogen (an energy-storage molecule in animal)
1.12 How are proteins synthesised?
by joining amino acids together, enzymes catalyse the reaction required to do this
1.12 What is the role of the pancreas in this?
to produce digestive enzymes and hormones that regulate blood sugar
1.13: CP How do you prepare a food sample?
-Break up the food using a pestle and mortar
-transfer to a test tube and add distilled water
-mix the food with water by stirring with a glass rod
-filter the mixture using a funnel and filter paper, collecting the solution
-proceed with food test
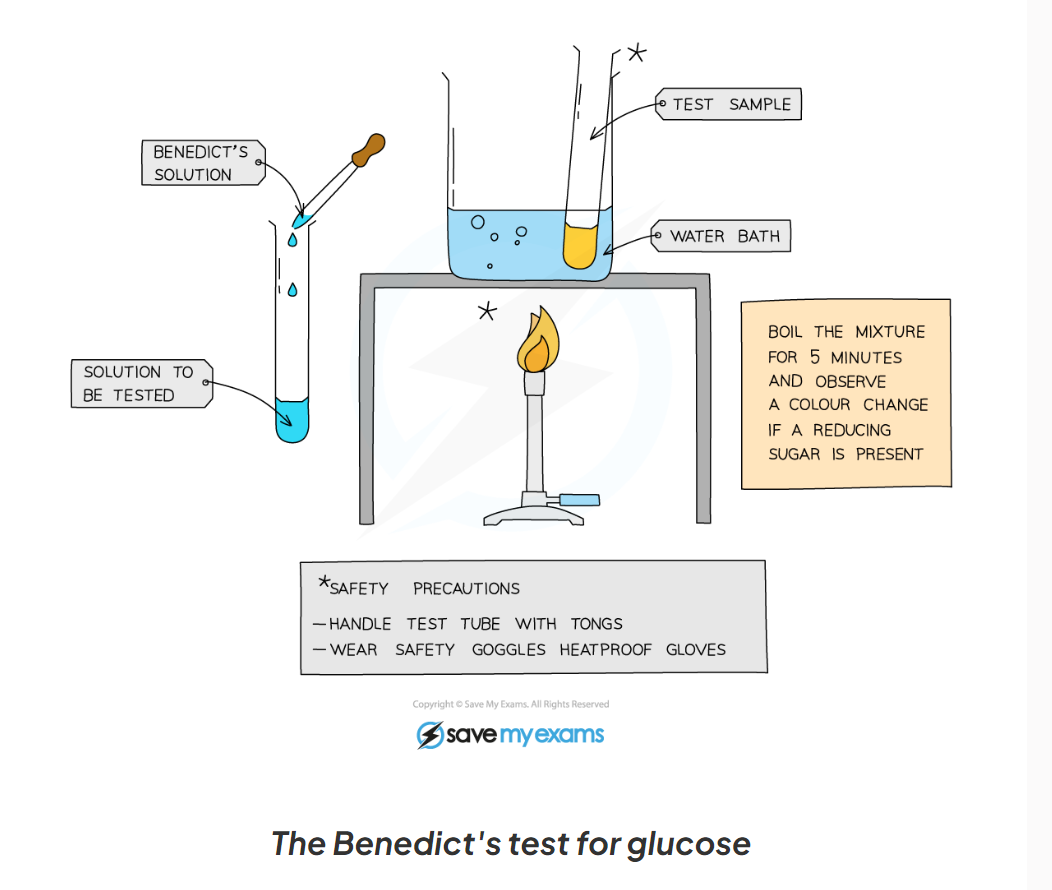
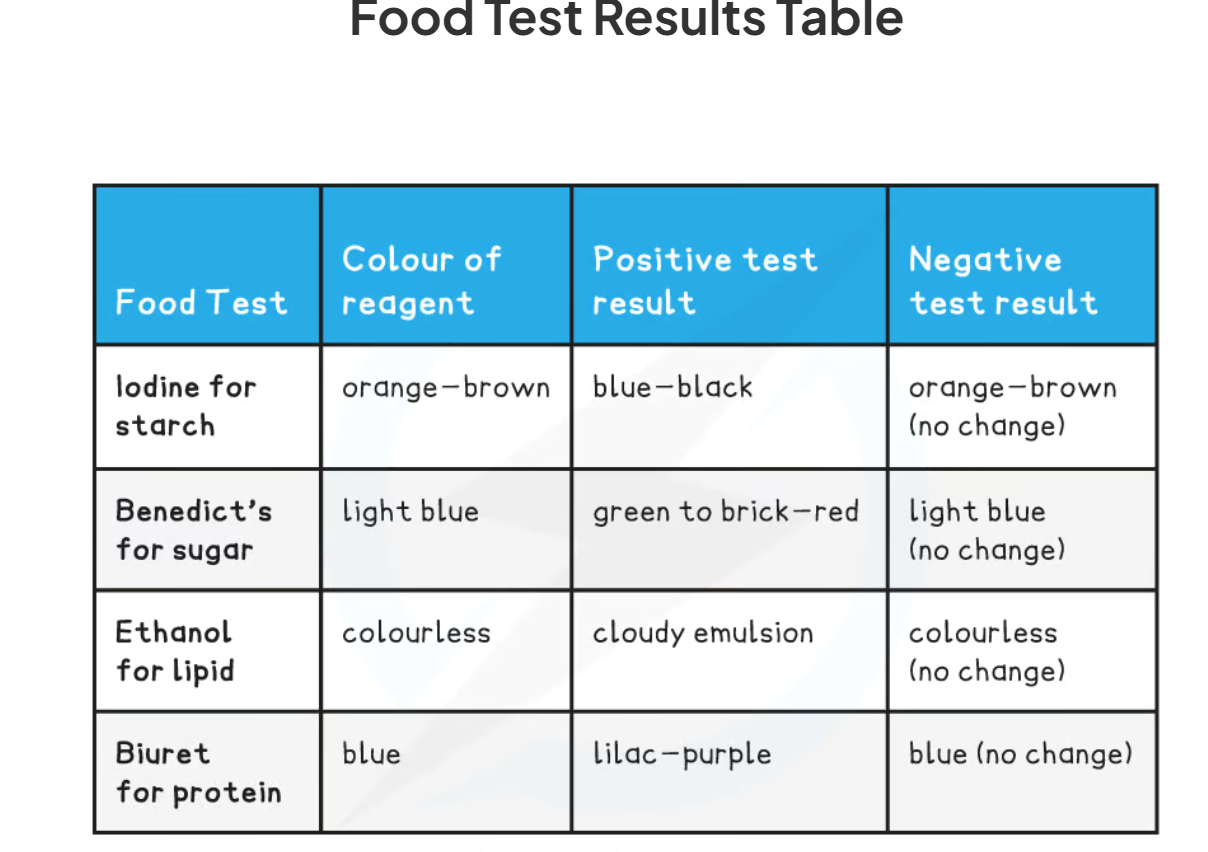
1.13: CP What is the process to test for glucose?
-add Benedict’s solution to the sample solution in a test tube
-heat in a boiling water bath for 5 minutes
-take the test tube out of the water bath and observe the colour
-a positive test will show a colour change from blue to orange/ brick red
1.13: CP Label this diagram of the apparatus needed for the Benedict's test for glucose
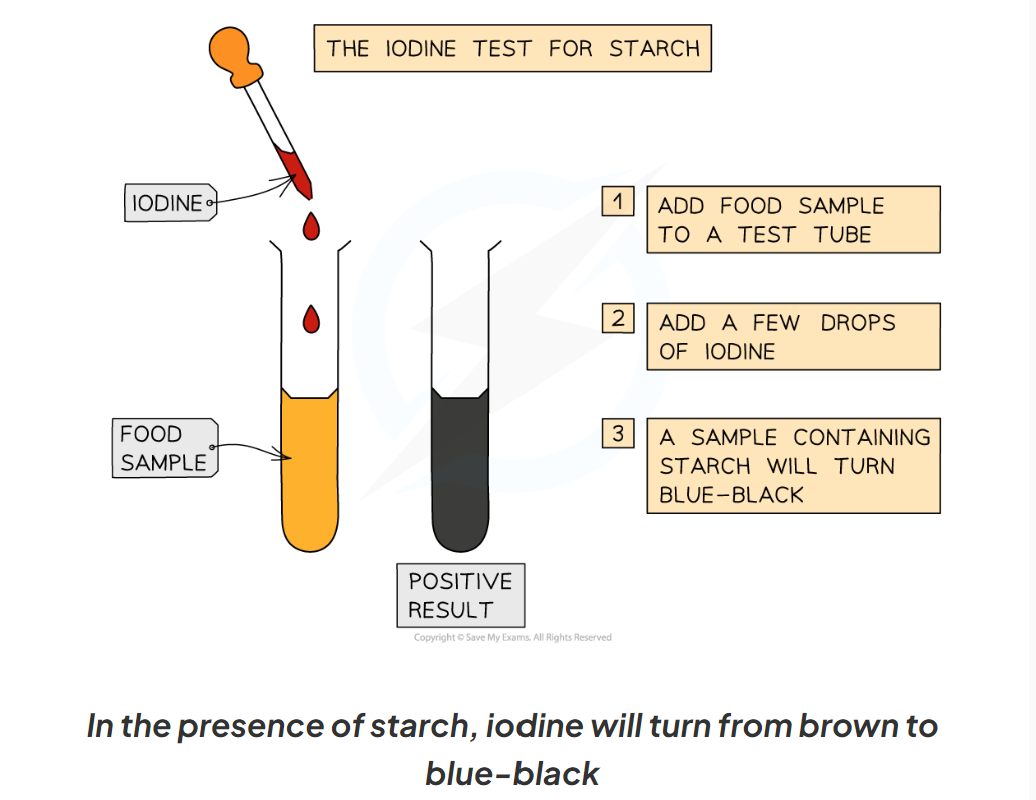
1.13: CP What is the process to test for starch?
-iodine can be used to test for starch
-add drops of iodine solution to the food sample (which you have already prepared)
-a positive test will show a colour change form orange-brown to blue-black
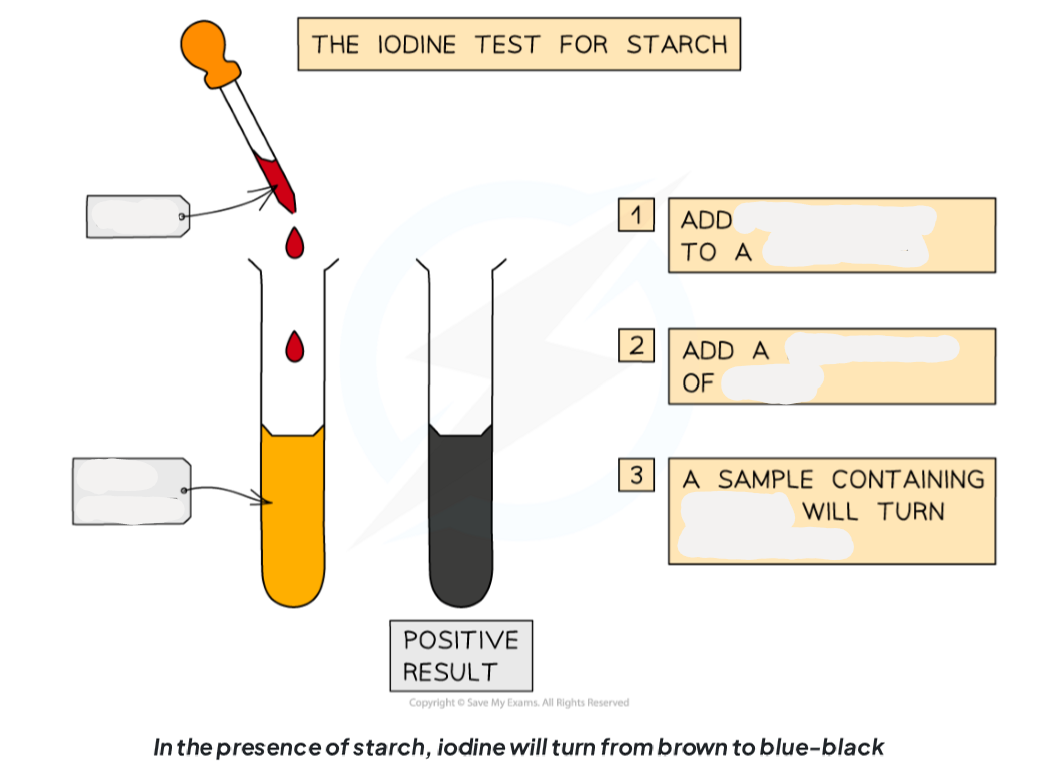
1.13: CP Label this diagram including the apparatus needed to test for starch
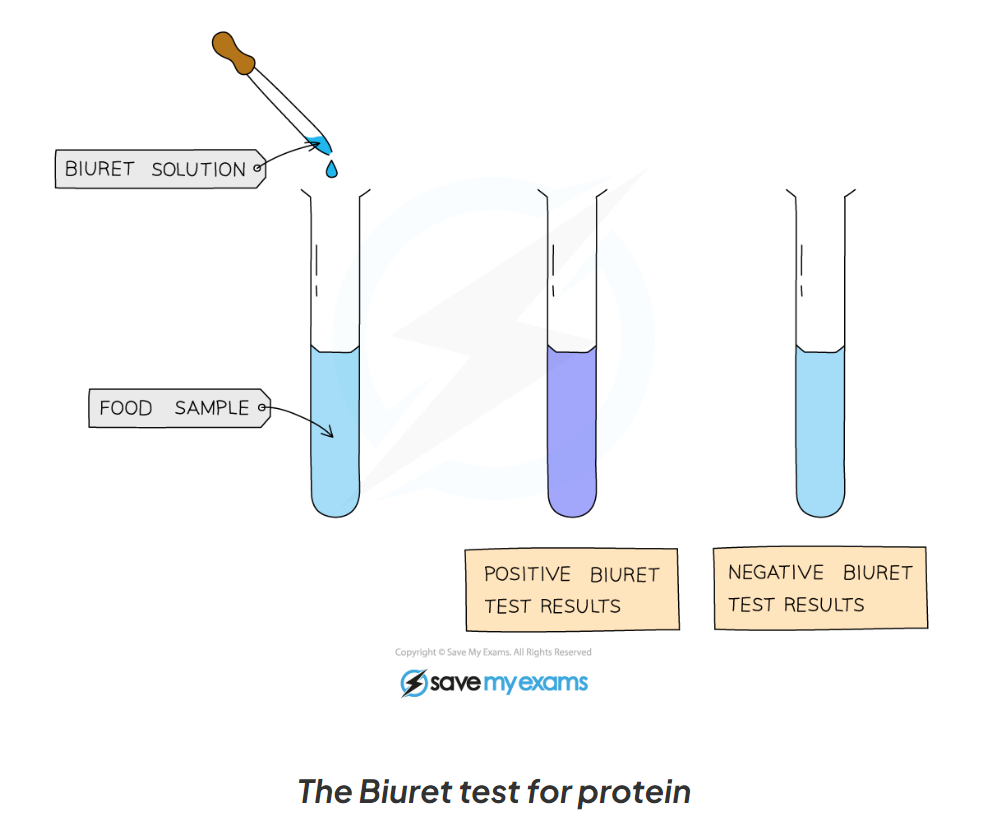
1.13: CP What is the process to test for protein?
-add drops of Biuret solution to the food sample
-a positive test will show a colour change from blue to violet/ purple
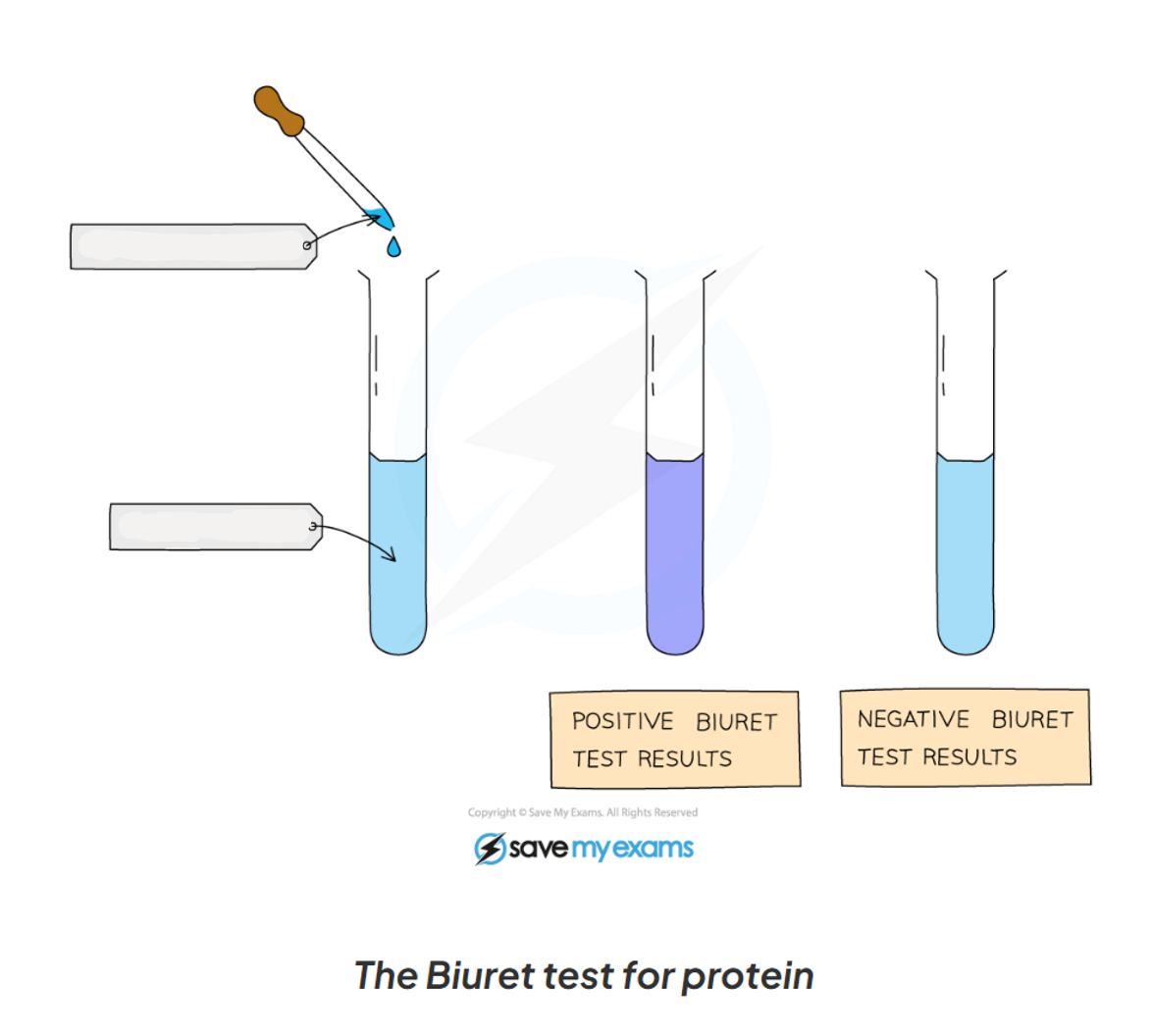
1.13: CP Label this diagram including the apparatus for the test for protein
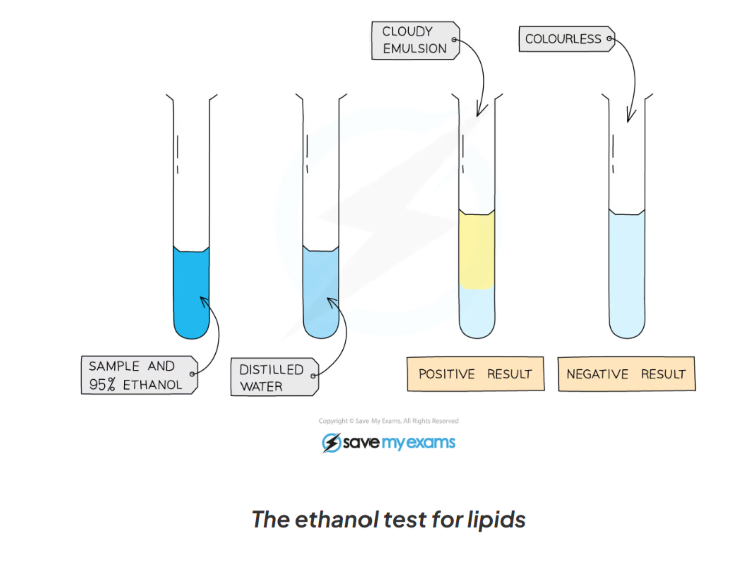
1.13: CP Explain the process to test for lipids
-mix the food sample with 4cm3 of ethanol and shake
-allow time for the sample to dissolve in ethanol
-strain the ethanol solution into another test tube
-add the ethanol solution to an equal volume of cold distilled water (4cm3)
-a positive test will show a cloudy emulsion forming
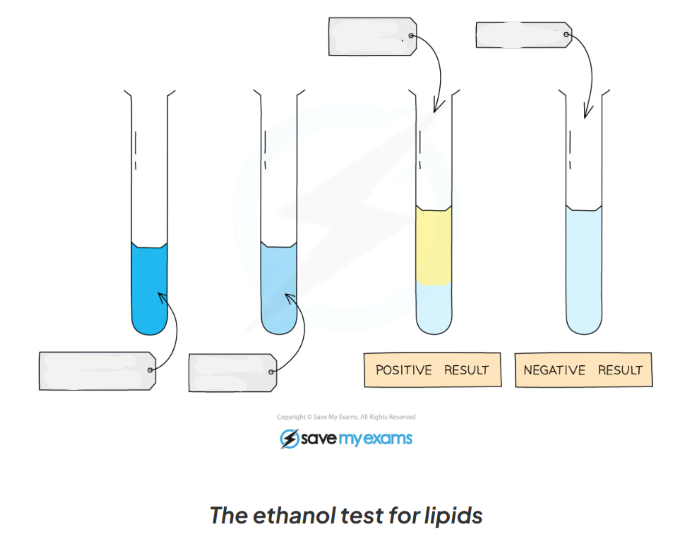
1.13: CP Label this diagram including the apparatus in the process of testing for lipids
1.13: CP Fill out this table
1.13: CP Give 4 important hazards to consider during this experiment
1) Biuret solution contains copper(ll) sulphate and iodine can be irritants to the eyes, so googles are needed
2) Sodium hydroxide is corrosive, so if it gets on your hands wash immediately
3) Ethanol is highly flammable: keep it away from the Bunsen burner
4) Keep fingers away from the Bunsen burner as it can be very hot
1.14 Explain the method for investigating the energy content of food in a simple calorimetry experiment
Use a measuring cylinder to measure out 25 cm3 of water and pour it into the boiling tube
Record the starting temp of the water using a thermometer
Set fire to the sample of food using a bunsen burner and hold the sample 2cm from the boiling tube until is has completely burned
Record the final temp of the water
Repeat the process with different food samples
E.g popcorn, nuts, crisps
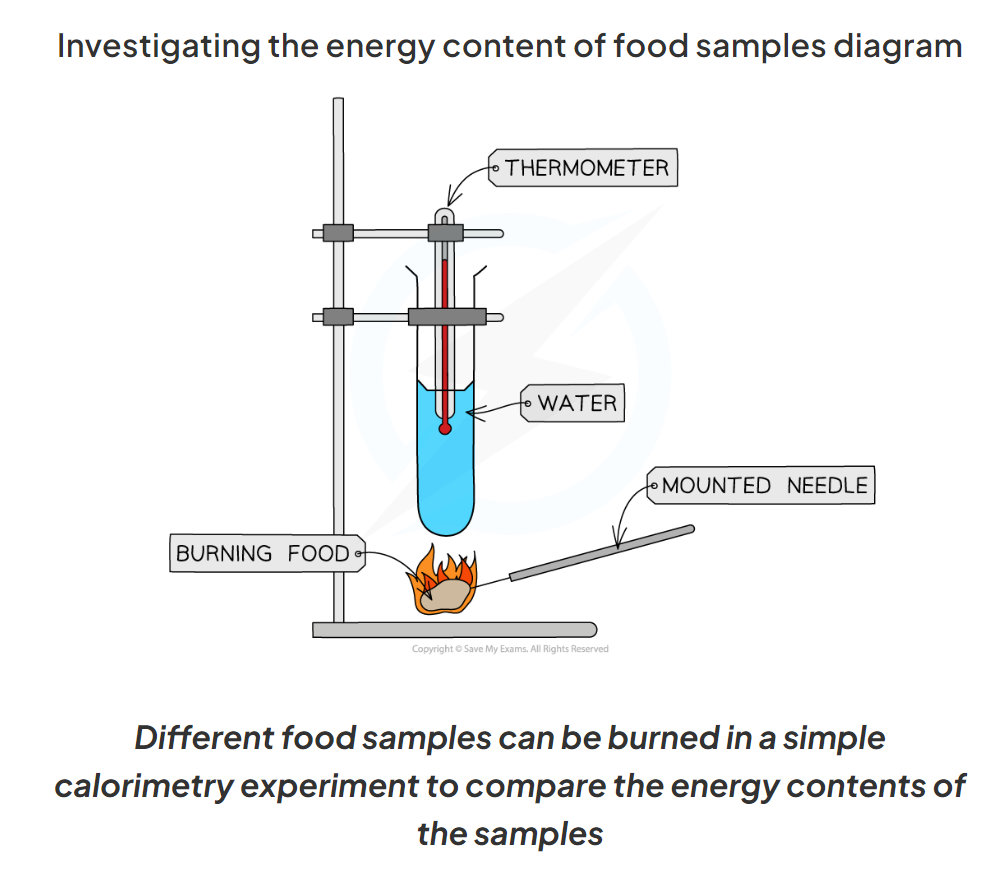
1.14 What are the results of this experiment which investigates the energy content of food samples?
-The larger the increase in water temp, the more energy is stored in the sample
1.14 What equation can be used to calculate the energy in each food sample?
-4.2 kJ is the specific heat capacity of water, meaning that it is the energy required to raise 1kg of water by 1°C
-1 cm3 of water has a mass of 1 g
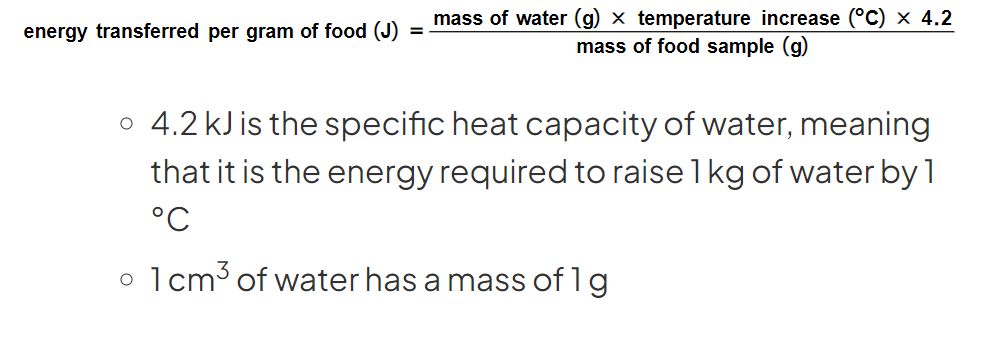
1.14 What are some limitations to the experiment which calculates the energy in food samples? And what is the solution#?
-incomplete burning of the food samples
Solution: Relight the food sample until it no longer lights up
-Heat energy is lost to the surroundings
Solution: Whilst heat lost meant that energy calculated is not very accurate, as long as the procedure is carried out in the exact same way each time (with the same distance between food samples and boiling tube), we can still compare results