antigen and antibody structure
1/18
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
19 Terms
What is an antigen?
An antigen is a molecule or part of a pathogen that is recognized by the immune system, triggering an immune response.
Difference between antigenicity and immunogenicity?
Antigenicity refers to the ability of a substance to bind to an antibody, while immunogenicity is the capacity to provoke an immune response, including the activation of T and B cells.
What is an antibody?
is a Y-shaped glycoprotein produced by B cells that binds specifically to antigens, neutralizing pathogens or marking them for destruction by the immune system.
Where are antibody produced?
Antibodies are produced in the bone marrow by plasma cells, which are differentiated B cells.
What stimulates antibody production?
The presence of antigens or pathogens, often in conjunction with helper T cells, stimulates antibody production.
What kind of response do they give?
A humoral immune response.
What is antibody function?
To bind, neutralize, and tag antigens for destruction.
What is an epitope?
A specific site on an antigen that is recognized by B-cell or T-cell receptors.
What are the different types of epitopes?
B-cell epitopes (surface-accessible) and T-cell epitopes (peptide fragments presented with MHC).
What type of structure may an epitope be made from?
Primary, secondary, tertiary, or quaternary structure; also polysaccharides.
What differences are there between B-cell receptor and T-cell receptor epitopes?
B-cell epitopes are accessible and native. T-cell epitopes are digested peptides presented by MHC
What are polyclonal antibodies?
Antibodies produced by different B-cell clones in response to multiple epitopes, resulting in a mixture of antibodies.
What is education in immunology, and why is it important?
It's the clonal selection process where self-reactive lymphocytes are eliminated, preventing autoimmunity.
What is the basic structure of an antibody?
Antibodies are Y-shaped proteins composed of two heavy chains and two light chains, connected by disulfide bonds, with variable regions
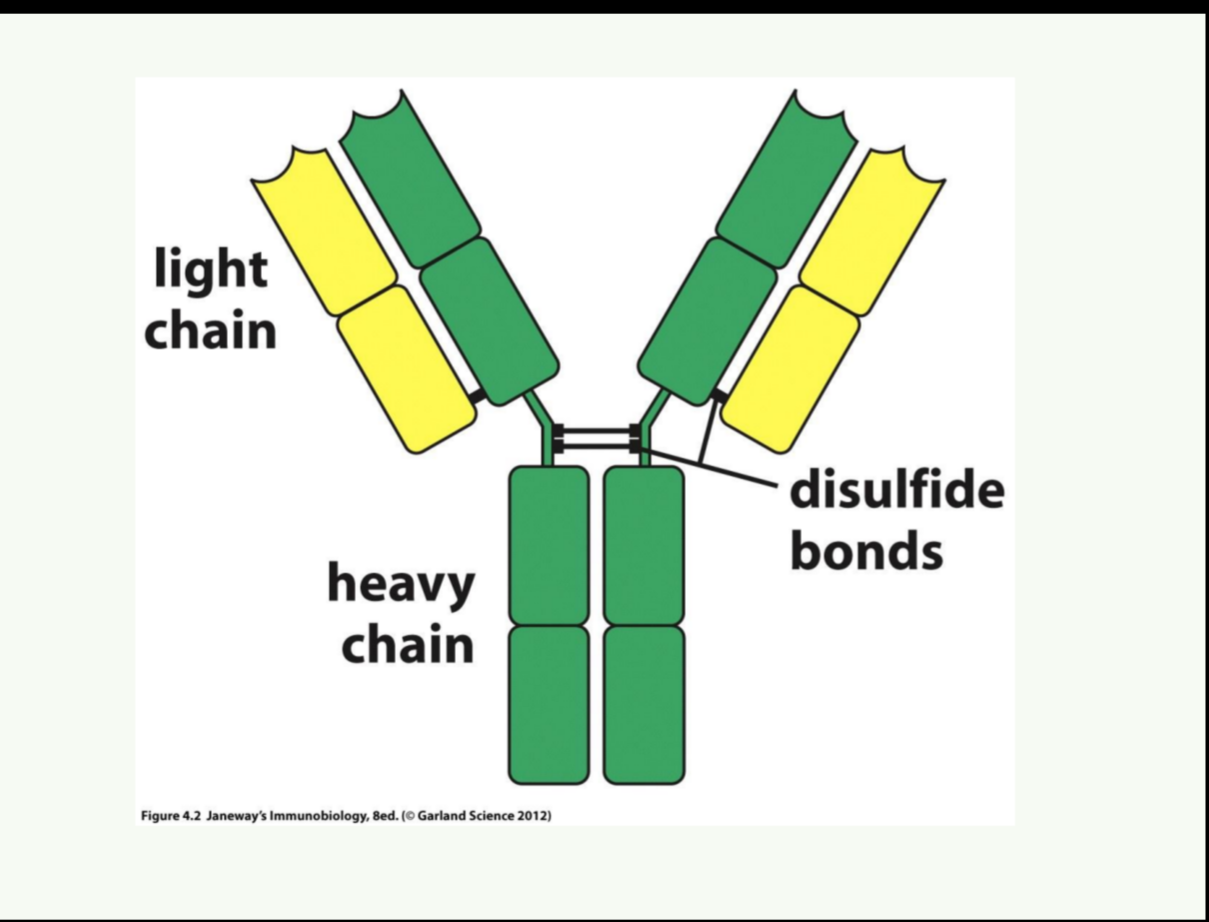
What are the different regions of an antibody?
Variable (V), constant (C), Fab (binding site), Fc (effector function), hinge region.
What are the different classes of antibody?
IgG, IgA, IgM, IgE, IgD
What determines these classes?
The type of heavy chain: γ (IgG), α (IgA), μ (IgM), ε (IgE), δ (IgD).
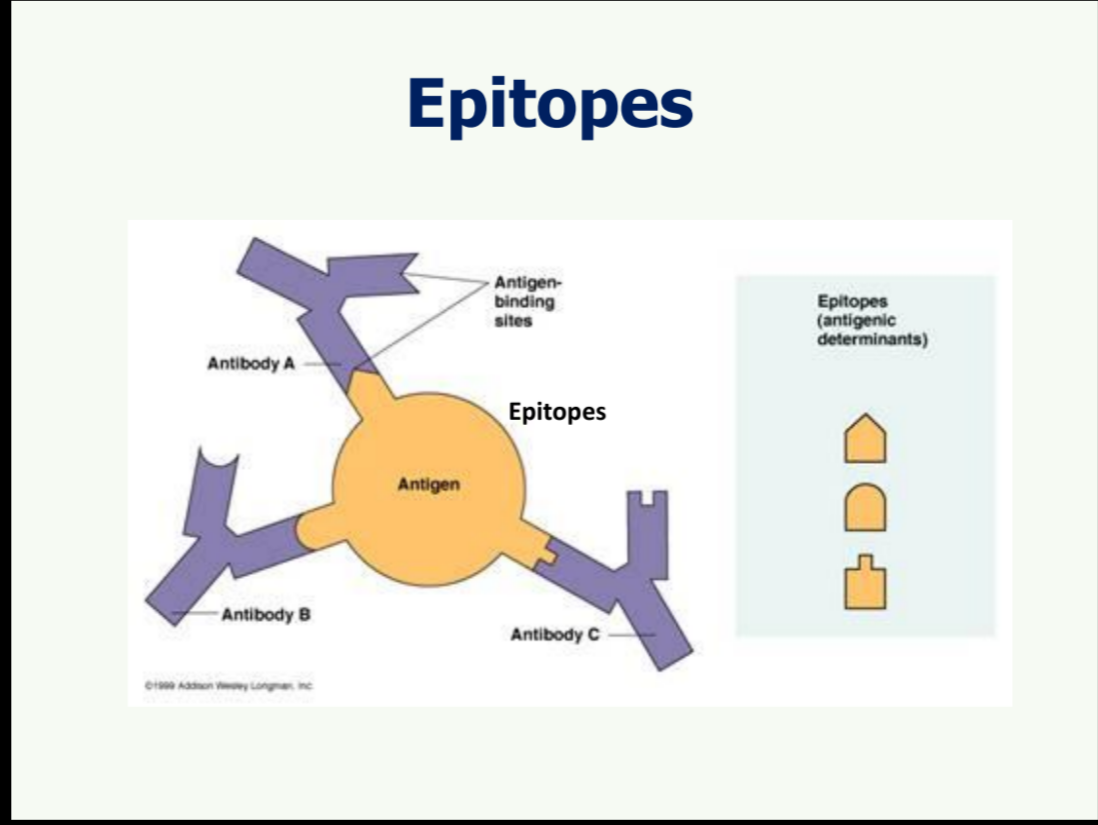
What is the structure of an antigen binding site?
Look at picture
What is the structure and function of the different classes of antibody?
IgG: Monomer, crosses placenta, activates complement.
IgA: Dimer/monomer, mucosal defense.
IgM: Pentamer, first responder.
IgE: Monomer, allergy & mast cell activation.
IgD: Monomer, B-cell receptor.