PAG 7.3 - Oxidation reactions of carbonyl compounds
1/4
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
5 Terms
Give the method for Tollens’ test
Prepare Tollens’ reagent: in a clean test tube add 3cm³ of aqueous silver nitrate, AgNO3 (aq)
Add aqeuous sodium hydroxide to the silver nitrate until a brown precipitate of silver oxide, Ag2O, is formed
Add dilute ammonia solution until the brown precipitate just dissolves to form a clear colourless solution. This is Tollens’ reagent
Pour 2 cm depth of the unknown solution into a clean test tube and add an equal volume of freshly prepared Tollens’ reagent
Leave the test tube to stand in a beaker of warm water of 50C and observe whether any silver mirror is formed

Why is Tollens’ reagent prepared immediately before carrying out the test?
It has a short shelf life
How should you dispose of all the ammoniacal silver nitrate solution and why?
Should be washed down the foul water drain with plenty of water
It must not be stored as silver filaments will form, which have been known to explode
Give the method using acidified potassium dichromate (VI) solution
Put 1 cm³ of acidified potassium dichromate (VI) solution into a clean test tube and add 10 drops of the solution
Warm gently using a hot water bath
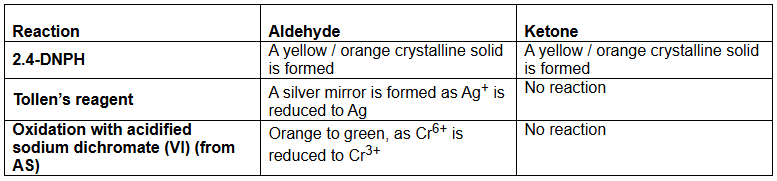
Give the results of the tests for distinguishing between aldehydes and ketones