Organic Chemistry Exam 1
1/111
Earn XP
Description and Tags
anything conceptual we need to know
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
112 Terms
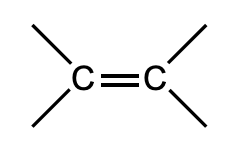
What is this functional group?
Alkene
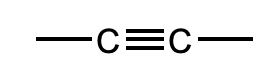
What is this functional group?
Alkyne
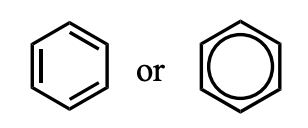
What is this functional group?
Benzene (an aromatic hydrocarbon)
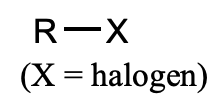
What is this functional group?
alkyl halide (haloalkane)
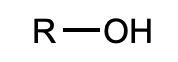
What is this functional group?
alcohol
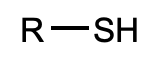
What is this functional group?
thiol
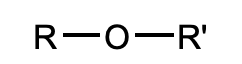
What is this functional group?
ether
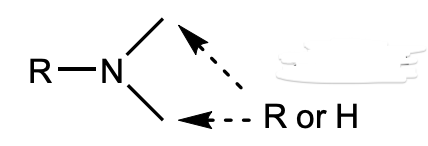
What is this functional group?
amine
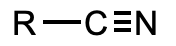
What is this functional group?
nitrile
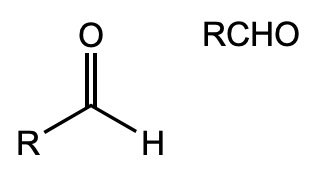
What is this functional group?
aldehyde
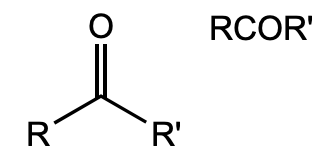
What is this functional group?
ketone
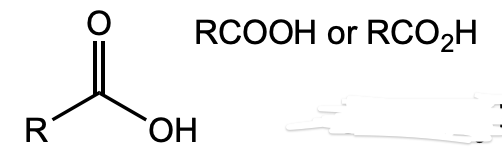
What is this functional group?
carboxylic acid

What is this functional group?
ester
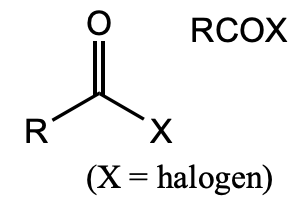
What is this functional group?
acid halide
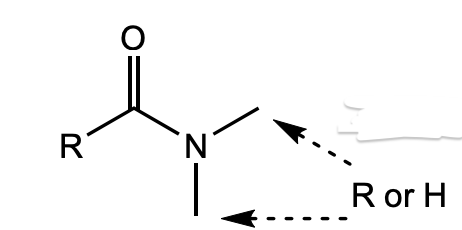
What is this functional group?
amide
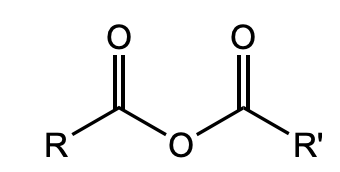
What is this functional group?
anhydride
pKa for HBr
-9
pKa for HCl
-7
pKa for H3O+
-1.7
pKa for HF
3.2
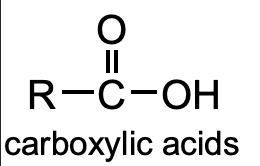
pKa for carboxylic acids
~4.7
pKa for NH4+ (H–NH3+)
9.2
pKa for alkyl ammonium ions
10-11
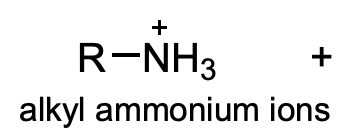
pKa for H2O (H–OH)
15.7
pKa for alcohols (R–OH)
16–18
pKa for NH3 (H–NH2) or amines (R–NH2)
~36
pKa for regular alkyl hydrogens
~50
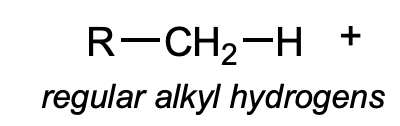
Bronsted-Lowry Acids and Bases
Acid: donates a proton
Base: accepts a proton
Lewis Acids and Bases
Acids: accepts a pair of electrons
Bases: donates a pair of non-bonding electrons
IUPAC Nomenclature - Alkanes and Alkyl Halides
find longest carbon chain in the molecule and name it according to the number of carbons in the chain. This is the parent chain. (if chains of equal length compete for selection, the one with more substituents win)
Number the carbons of the parent chain so that the numbers of the substituent positions are as low as possible.
Name the substituents. All substituents receive numbers that indicate their positions in the parent chain. Multiple identical substituents require prefixes (di, tri, etc)
Non-identical substituents are ordered alphabetically. Prefixes are not considered in alphabetical order, but “iso” is considered.
If 2 numbering directions give the same set of numbers for the substituents, the correct direction is the one that gives the lowest number to the substituent that appears first alphabetically.
when the numbering direction isn’t easily determined from previous rules, apply the “first point of difference” rule.
Methane
1 carbon
CH4
Ethane
2 carbon chain
CH3CH3
Propane
3 carbon chain
CH3CH2CH3
Butane
4 carbon chain
CH3CH2CH2CH3
Pentane
5 carbon chain
CH3(CH2)3CH3
Hexane
6 carbon chain
CH3(CH2)4CH3
Heptane
7 carbon chain
CH3(CH2)5CH3
Octane
8 carbon chain
CH3(CH2)6CH3
Nonane
9 carbon chain
CH3(CH2)7CH3
Decane
10 carbon chain
CH3(CH2)8CH3
Undecane
11 carbon chain
CH3(CH2)9CH3
Dodecane
12 carbon chain
CH3(CH2)10CH3
Tridecane
13 carbon chain
CH3(CH2)11CH3
Prefixes for two or more of the same substituent
di = 2
tri = 3
tetra = 4
penta = 5
hexa = 6
hepta = 7
octa = 8
nona = 9
Halogen Groups (substituents)
F = fluoro
Cl = chloro
Br = bromo
I = iodo
Cycloalkanes
cyclopropane
cyclobutane
cyclopentane
cyclohexane
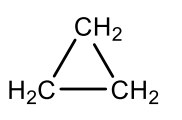
Which cycloalkane is this?
cyclopropane
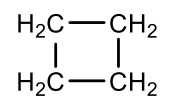
Which cycloalkane is this?
cyclobutane
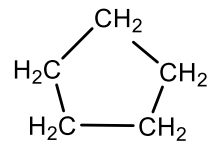
Which cycloalkane is this?
cyclopentane
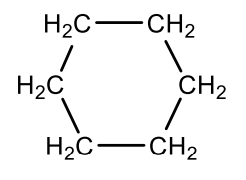
Which cycloalkane is this?
cyclohexane
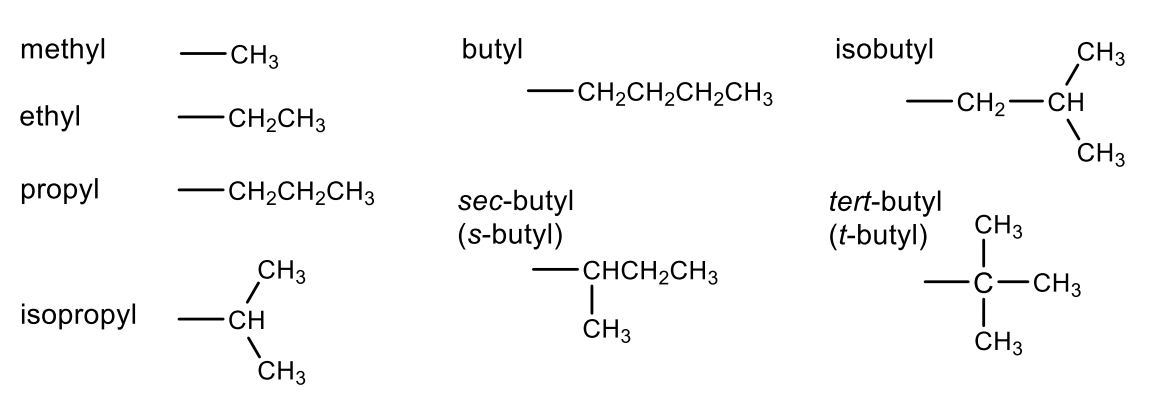
Alkyl Groups (substituents)
methyl
ethyl
propyl
isopropyl
butyl
sec-butyl (s-butyl)
isobutyl
tert-butyl (t-butyl)
CH3Br
common name: methyl bromide
IUPAC name: bromomethane
CH2Cl2
common name: methylene chloride
IUPAC name: dichloromethane
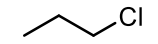
CH3CH2CH2Cl
common name: propyl chloride
IUPAC name: 1-chloropentane
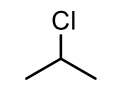
CH3CHClCH3
common name: isopropyl chloride
IUPAC name: 2-chloropropane
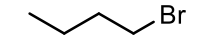
CH3CH2Ch2CH2Br
common name: n-butyl bromide (butyl bromide)
IUPAC name: 1-bromobutane
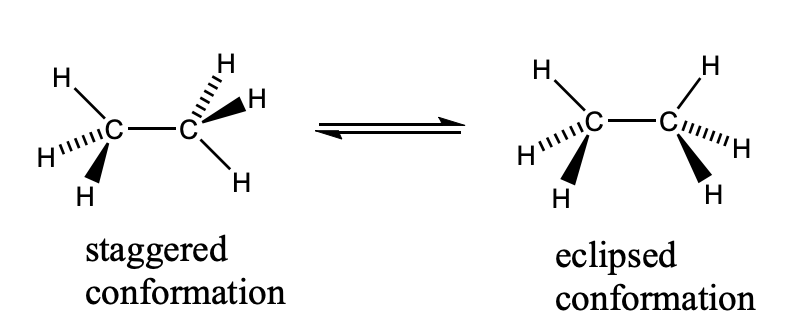
Newman Projections
first image is the lewis structure
second is Newman projections
staggered conformation = most stable, lowest energy
eclipsed conformation = least stable, highest energy
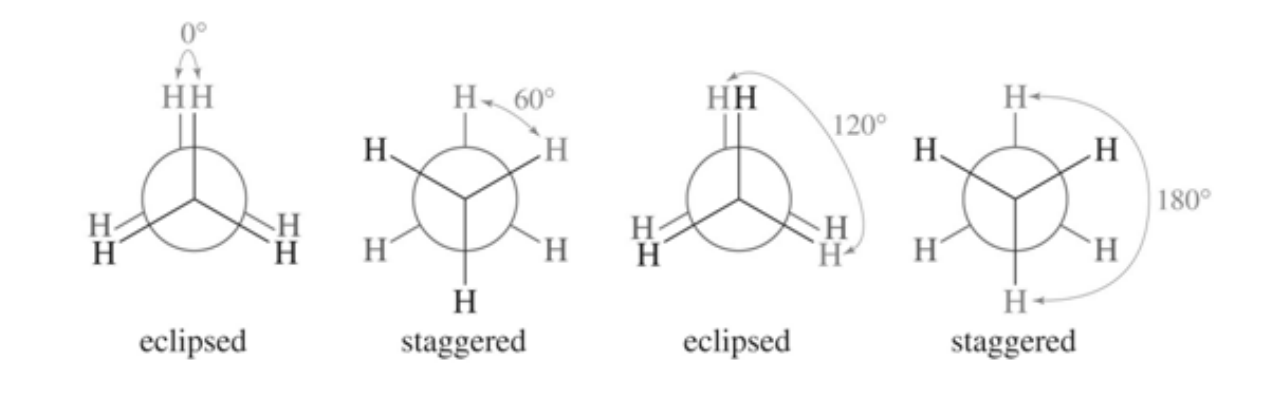
conformations
because the C-C single bond can rotate, this molecule can have many conformations (different 3D arrangements formed by rotation of a single bond)
Torsional strain
destabilization caused by the eclipsing of bonds on neighboring atoms (the eclipsing bonds on neighboring atoms experience repulsion because of the electron in the bonds)
Steric strain (steric hindrance)
the interference between two groups that are so close together that their electron clouds experience a repulsion (destabilizing)
Stability of Newman projections
least, to most stable
totally eclipsed < eclipsed < gauche < anti
Cis-Trans Isomerism in Cycloalkanes
the carbon-carbon single bonds in a ring cannot freely rotate, so cis-trans isomerism is possible for certain substituted cycloalkanes.
stereoisomers for cycloalkanes
stereoisomers - isomers that have the same connectivity of atoms but different spatial arrangements
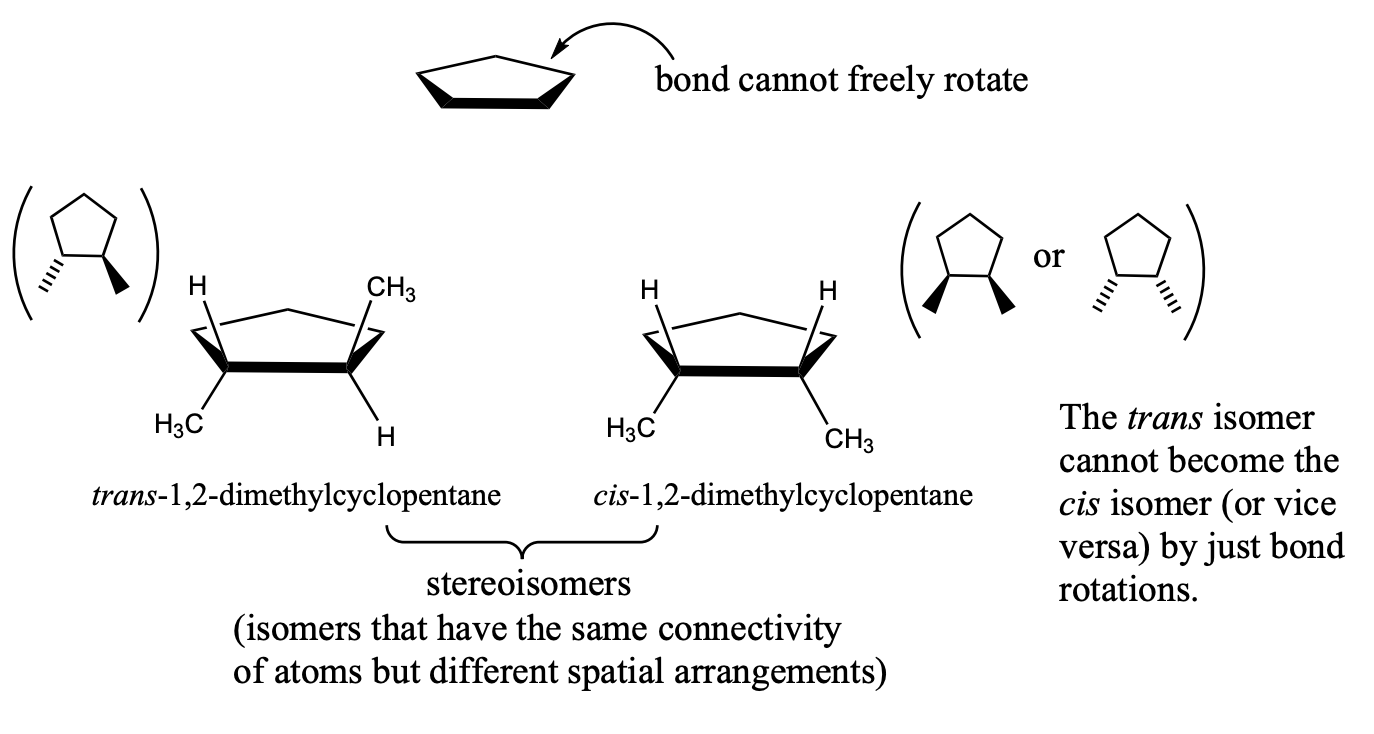
London Dispersion Forces
attractive forces resulting from temporary dipole moments induced in a molecule by other nearby molecules
These attractive forces are roughly proportional to the molecular surface area. The more molecular surface area, the stronger the forces.
dipole-dipole forces
attractive forces resulting from the permanent dipole moments of polar molecules
Polar compounds generally have higher boiling points than non-polar ones.
Acid Strength
expressed by the extent of the acid’s ionization in water
smaller the pKa, the stronger the acid
a strong acid has a weak conjugate base
a weak acid has a strong conjugate base
Which is a stronger base, CH3O- or Cl- ?
CH3O because
CH3OH has an OH which is a strong base
Also the conjugate acid for Cl- is HCl which is a strong acid, meaning the conjugate base is weak.
Which is a stronger base, Cl- or F- ?
F- because HF is a weak acid, therefore, strong conjugate base
How does an acid-base reaction favor reactants and products?
they favor the weaker acid and the weaker base
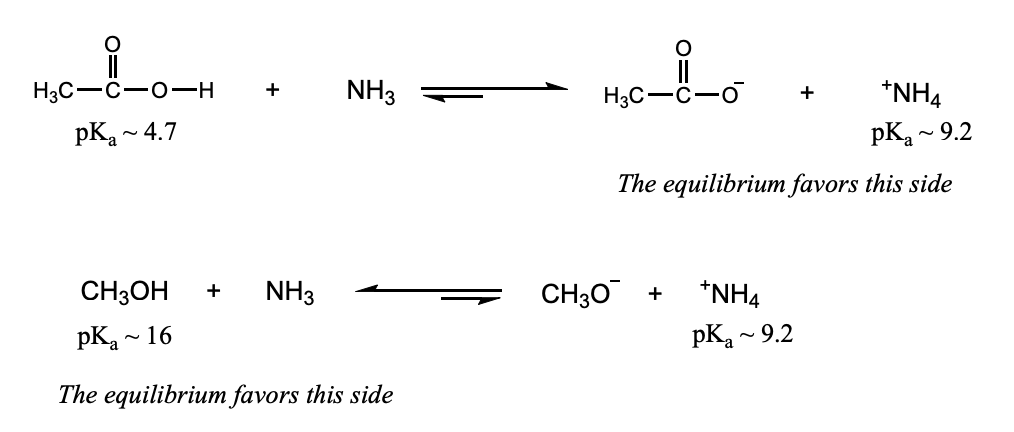
Factors Affecting Acidity: Electronegativity
Electronegativity: a more electronegative element bears a negative charge more easily, giving a more stable conjugate base. The corresponding acid is stronger.
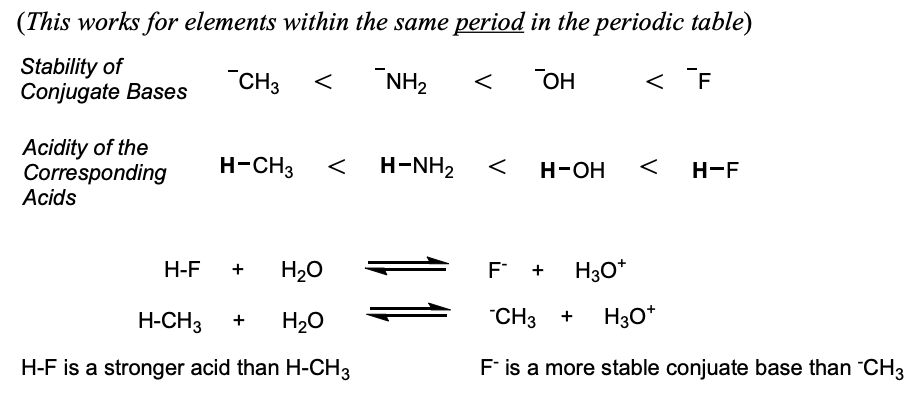
relationship between conjugate bases and acid strength
more stable the conjugate base, the stronger the acid
more stable the base, the weaker the base
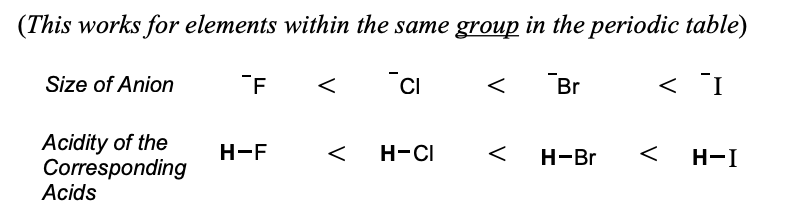
Factors Affecting Acidity: Size of Anions
The negative charge of an anion is more stable if it is spread over a larger region of space
When we compare the elements in the same group in the periodic table, the size of the anion is more important than the electronegativity of the elements.
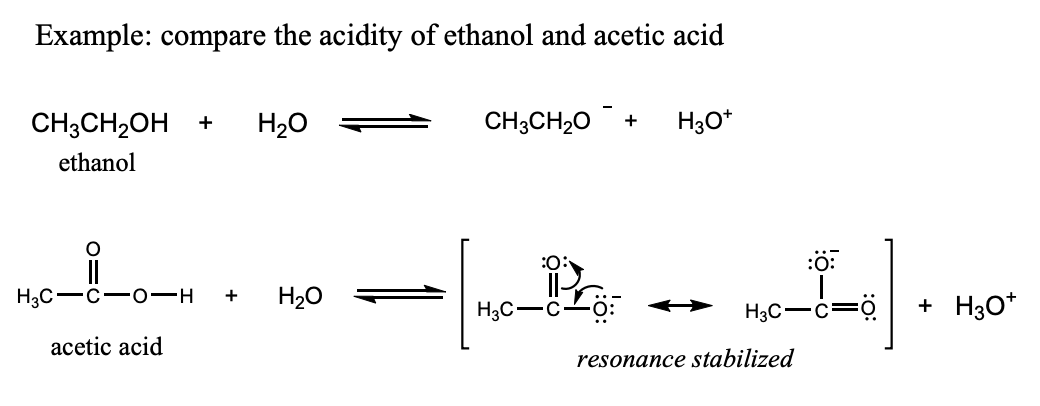
Factors Affecting Acidity: Resonance Stabilization
Acetic acid is a stronger acid than ethanol because the conjugate base of acetic acid is stabilized by resonance.
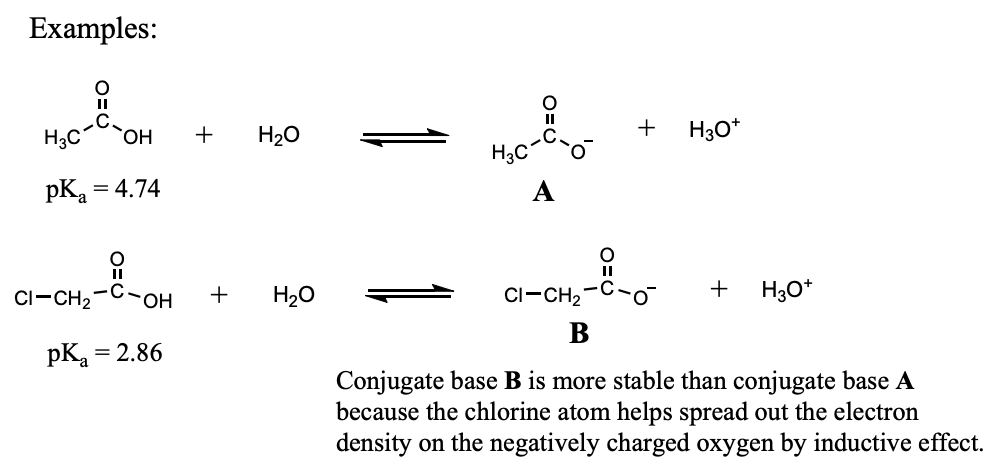
Factors Affecting Acidity: Inductive Effect
Inductive effect: Electron donation or withdrawal through the sigma bonds of a molecule.
Inductive effect from an electron-withdrawing group helps stabilize a conjugate base by "pulling" electron density away from an area of high electron density (helps spread out the electron density).
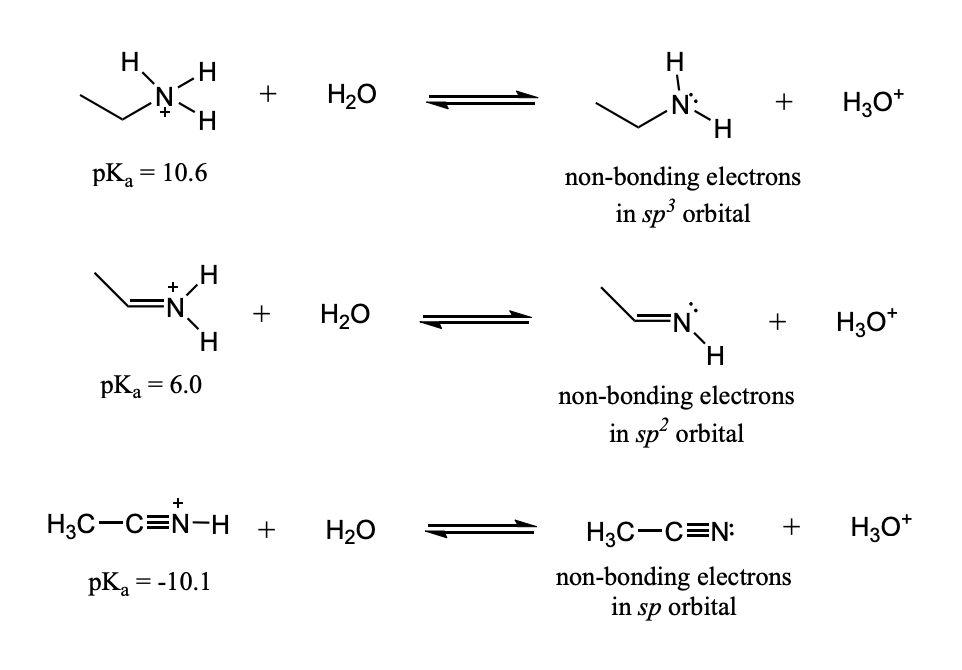
Factors Affecting Acidity: Hybridization
The non-bonding electrons remaining in a hybrid orbital after an acid has donated a proton are more stable in a hybrid orbital using more of the s orbital and less of the p orbitals.
non-bonding electrons are more stable when they are in:
sp orbital > > sp2 orbital > sp3 orbital
because the 2s orbital is lower than the 2p orbital
Therefore, an sp orbital is lower in energy than an sp2 orbital, and an sp2 orbital is lower in energy than an sp3 orbital.
formal charge formula
formal charge = [valence electrons] - [nonbonding electrons] - ½ [shared electrons]
Resonance Structures
different electronic representations of a chemical structure used to give a better description of the molecule
delocalization of electrons or charges through resonance is a stabilizing factor
Each resonance structure contributes to the real structure of the molecule, but some contribute more. (Not all resonance structures of a molecule are equally important.)

When drawing resonance structures
Don't move any atoms.
Move only electrons (usually or lone-pair electrons).
Keep track of formal charges on the atoms (all resonance structures of a molecule must have the same net charge).
Make sure all resonance structures are valid Lewis structures (pay attention to the "octet rule").
In general, resonance structures with more than two non-zero formal charges or one of the atoms having a 2+ or 2- charge are not considered to be important. We usually do not draw these resonance structures.
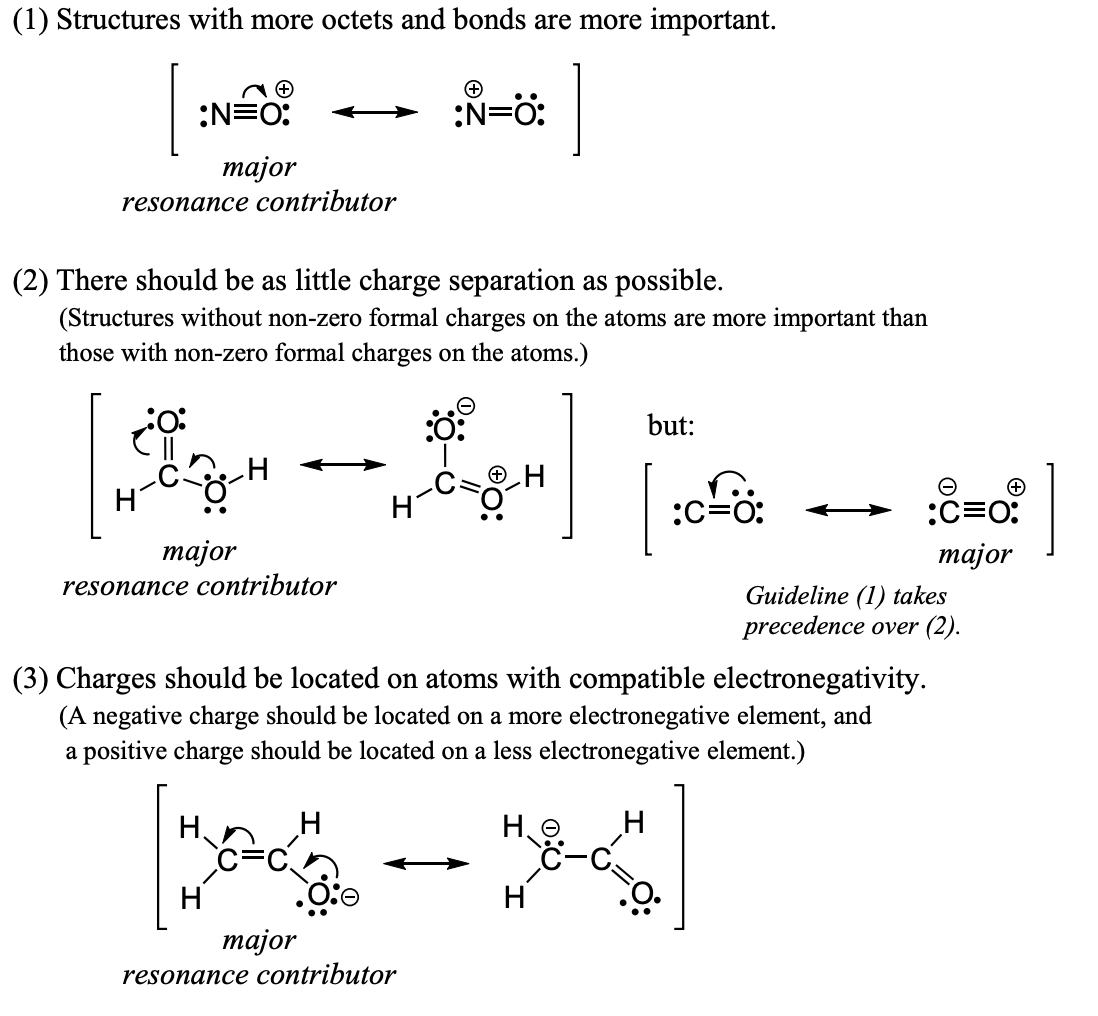
Guidelines for telling which resonance structures are more important
structures with more octets and bonds are more important
there should be as little charge separation as possible
charges should be located on atoms with compatible electronegativity
what are the 3 different ways of writing chemical structures?
lewis structure
condensed structure
Line-Angle structure
Each "angle" is a carbon. Each "end" is a carbon (unless stated otherwise). The hydrogens on the carbons are usually not shown. [It is common practice to show the hydrogens attached to oxygen, nitrogen, or sulfur.]
![<ul><li><p>lewis structure</p></li><li><p>condensed structure</p></li><li><p>Line-Angle structure</p><ul><li><p>Each "angle" is a carbon. Each "end" is a carbon (unless stated otherwise). The hydrogens on the carbons are usually not shown. [It is common practice to show the hydrogens attached to oxygen, nitrogen, or sulfur.]</p></li></ul></li></ul><p></p>](https://knowt-user-attachments.s3.amazonaws.com/8576bfd3-b1bd-4e2e-95dd-9206d281bcc0.png)
Molecular orbitals
A sigma (𝝈) bond is a bond with most of its electron density centered along the line joining the nuclei. It is a cylindrically symmetrical bond.
A pi (π) bond is formed by the overlap of two p orbitals oriented perpendicular to the line joining the nuclei. A pi bond has its electron density in two lobes, one above and one below the line joining the nuclei
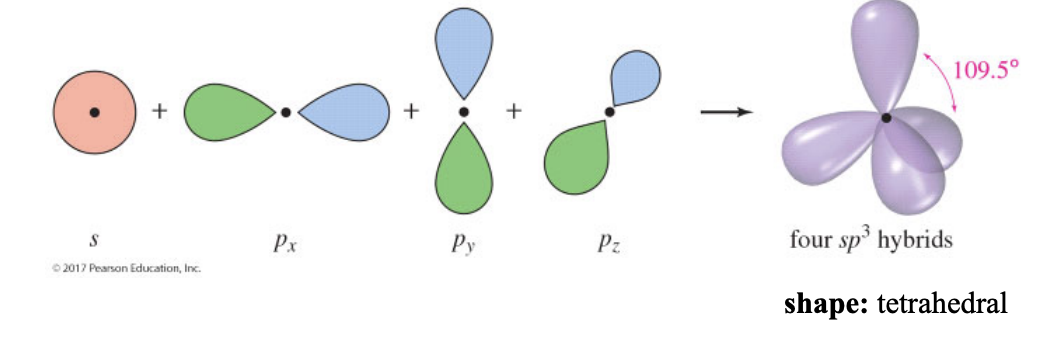
sp3 hybridization
the carbon atom has 4 electron domains around it
shape: tetrahedral
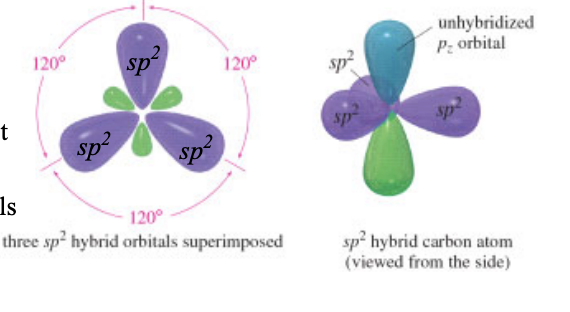
sp2 hybridization
each of the carbon atoms has 3 electron domains
shape: trigonal planar
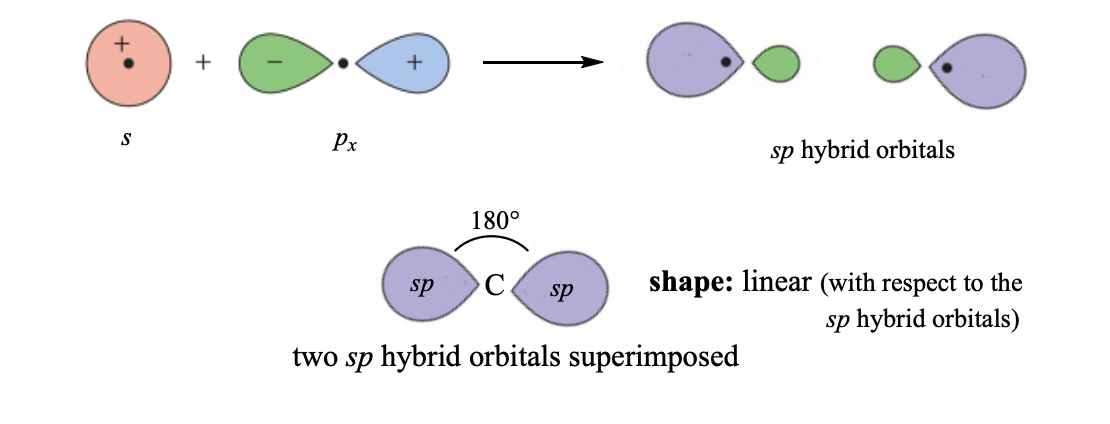
sp hybridization
each of the carbon atoms has 2 electron domains
shape: linear
Isomerism
isomers are different compound with the same molecular formula
constitutional isomers (structural isomers)
compounds of the same molecular formula but different structures (the atoms are connected differently)
Constitutional isomers are different compounds with different physical properties.
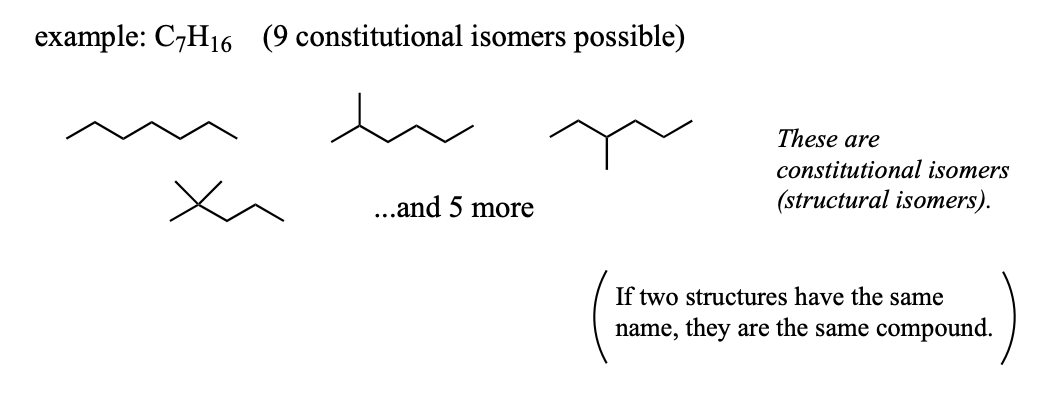
Stereoisomers
isomers that have the same connectivity of atoms but different spatial arrangements.
geometric isomers
stereoisomers that have the same connectivity of atoms but different spatial arrangements due to the presence of a carbon-carbon double bond.
cis = hydrogens on the same side of the double bond
trans = hydrogens on the opposite sides of the double bond
geometric isomers have different physical properties
to have geometric isomerism, a compound must have two different groups on each of the carbons that are bonded with a double bond
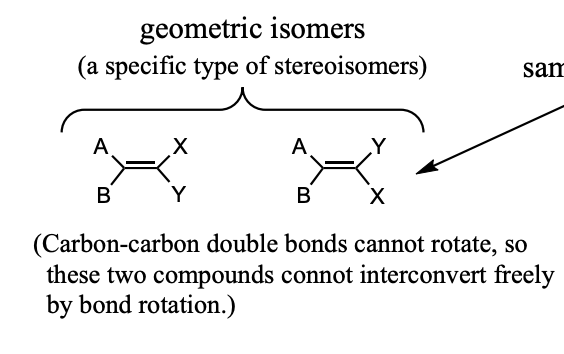
How to know when two compounds are the same
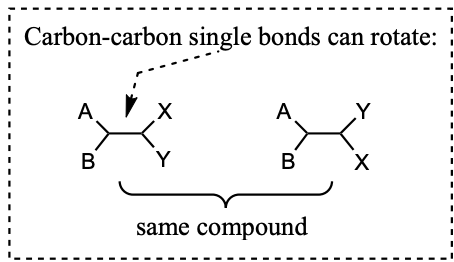
Carbon-Carbon single bonds can freely rotate
stability of cycloalkanes
sp3 hybridized carbon (tetrahedron) with a 109.5° bond angle is ideal for optimal stability
If a carbon atom cannot achieve this ideal bond angle, there will be angle strain in the molecule.
angle strain
destabilization caused by the deviation from the ideal bond angle.
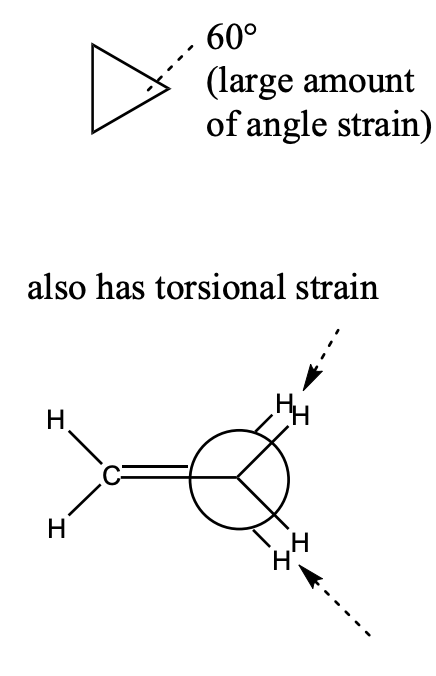
cyclopropane
60° bond angles
large amt angle strain
torsional strain
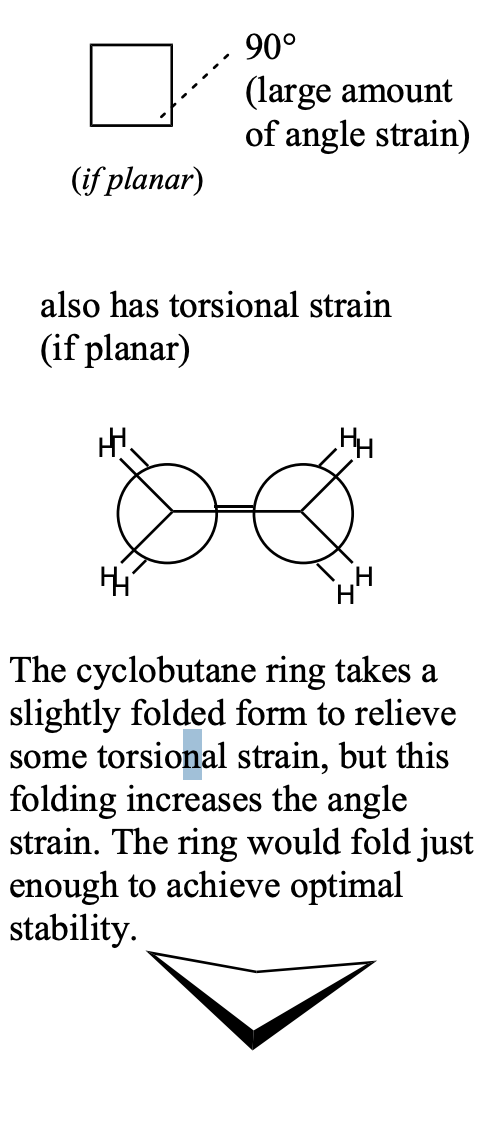
cyclobutane
90° bond angles
large amount angle strain
torsional strain if planar
The cyclobutane ring takes a slightly folded form to relieve some torsional strain, but this folding increases the angle strain. The ring would fold just enough to achieve optimal stability
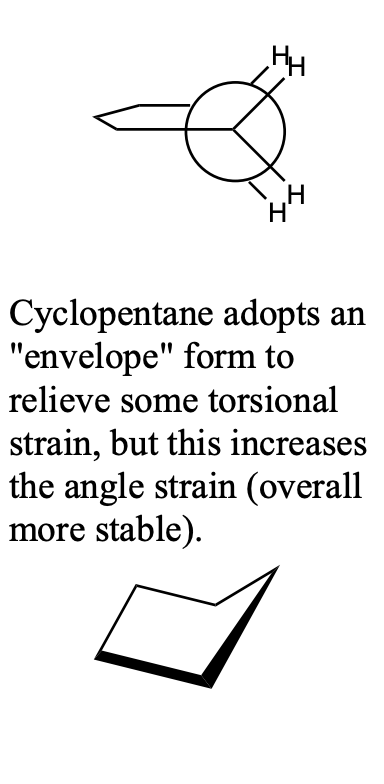
cyclopentane
108° bond angles
small amount of angle strain
also torsional strain if planar
Cyclopentane adopts an "envelope" form to relieve some torsional strain, but this increases the angle strain (overall more stable).
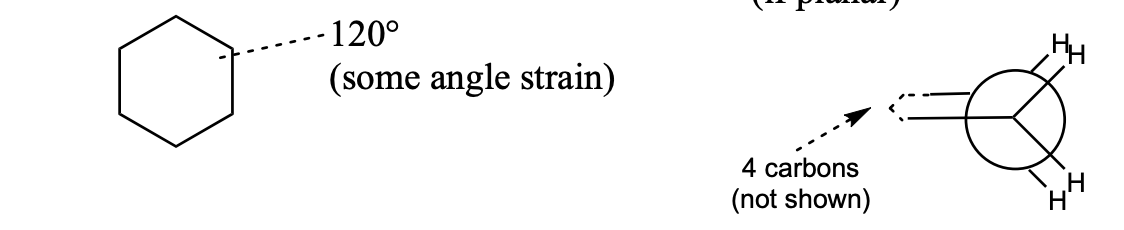
cyclohexane
if planar
120° → some angle strain
torsional strain
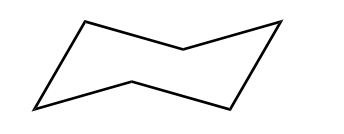
Cyclohexane adopts a "chair" conformation to remove almost all angle strain and torsional strain.
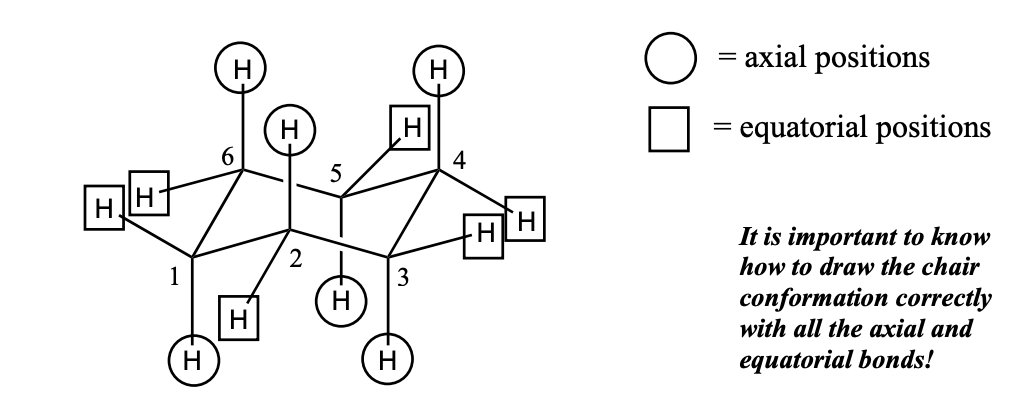
chair conformation of cyclohexane
6 axial positions (3 “up” and 3 “down”)
6 equatorial positions (3 “up” and 3 “down”)
It is important to know how to draw the chair conformation correctly with all the axial and equatorial bonds!
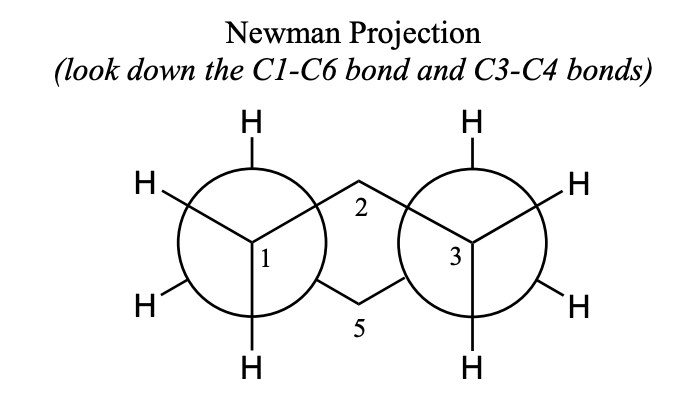
newman projection of chair conformation of cyclohexane
Drawing chair conformations
only use solid lines
it’s not usually necessary to show all the hydrogens on the ring
Practice drawing these chair conformations with the axial and equatorial bonds. Make sure the bonds are pointing at the correct directions.
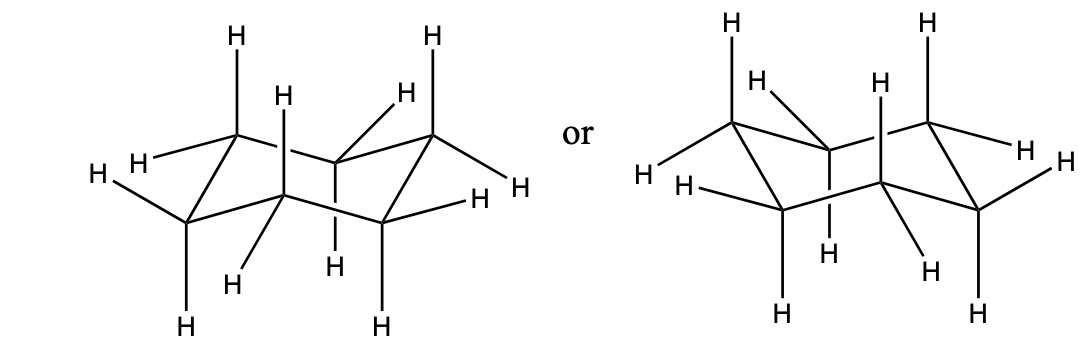
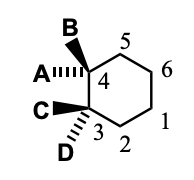
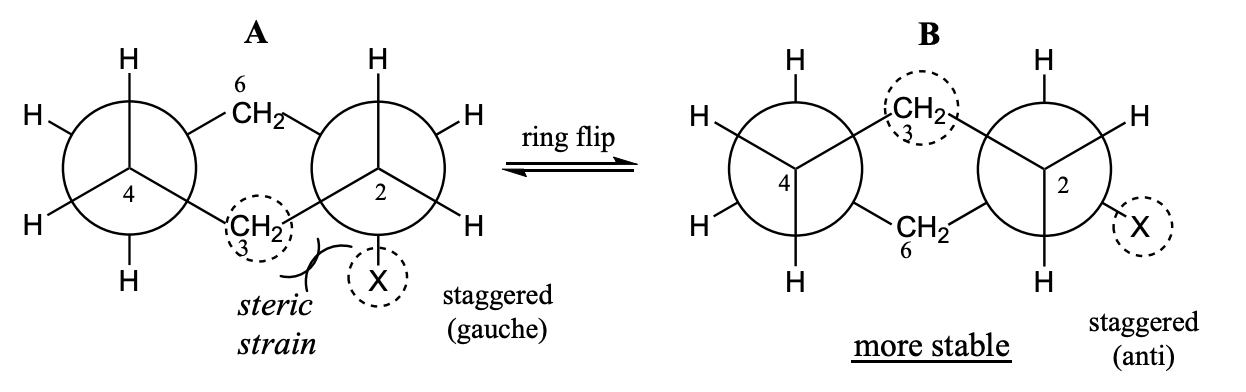
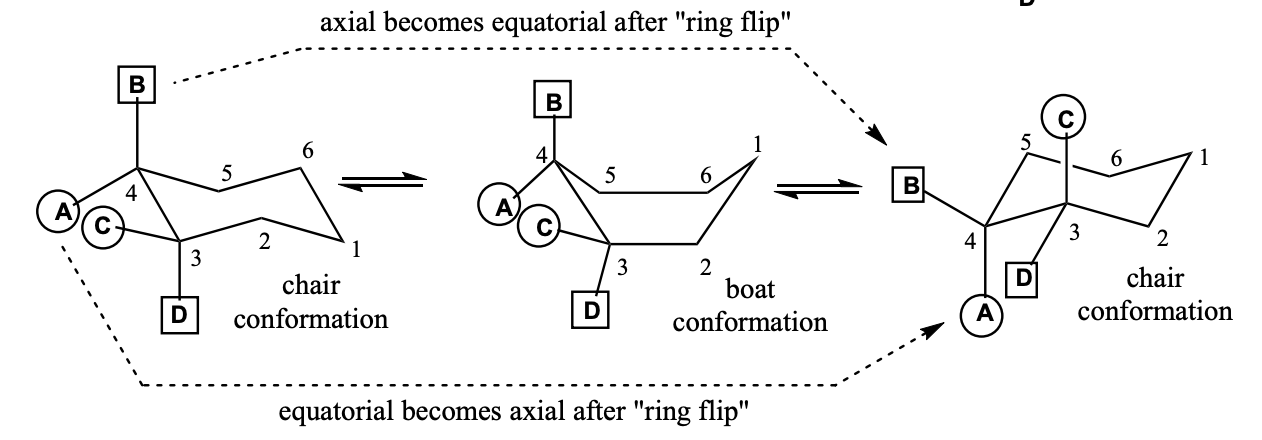
Chair-Chair Interconversion (“ring flip”)
After a ring flip, all the previous equatorial positions now become axial, and all the previous axial positions are now equatorial.
After a ring flip, all the previous "up" positions will stay "up", and all the previous "down" positions will stay "down".
A & C point away from ring, B & D point up and down
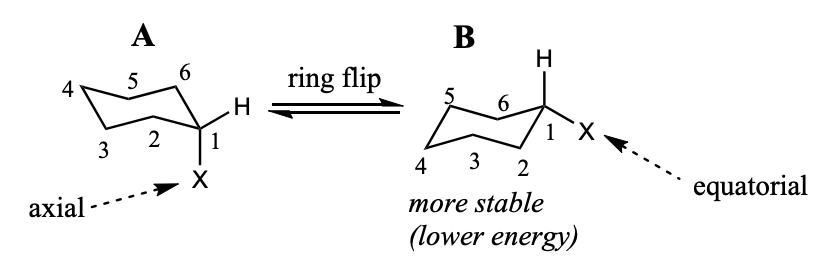
Conformations of monosubstituted cyclohexanes
For monosubstituted cyclohexanes, the chair conformation with the substituent at the equatorial position is more stable.