EL 5 The molecules of life
1/29
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
30 Terms
Dative covalent bond
both electrons come from the same atom
How to draw molecules with stick and wedge notation
straight lines represent bonds on the plane of the paper
solid wedge lines represent bonds coming towards you
dashed wedge lines represent bonds going away from you
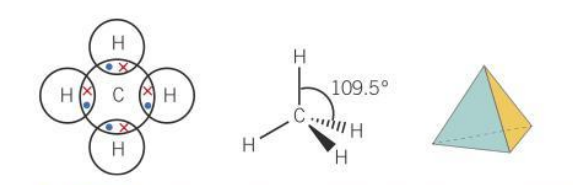
Bond angle for a tetrahedral molecule
109.5
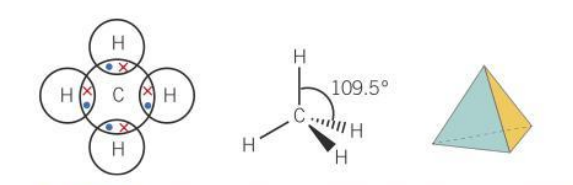
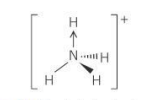
bond angle for ammonium ion
109.5
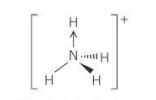
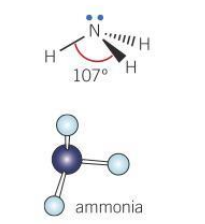
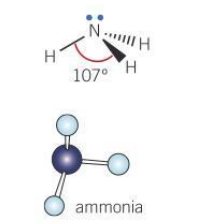
Bond angle for a pyramidal molecule
107
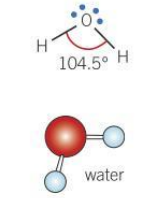
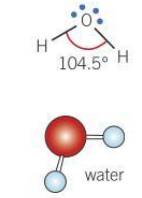
Bond angle for a bent molecule
104.5
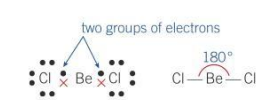
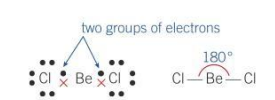
Bond angle for a linear molecule
180
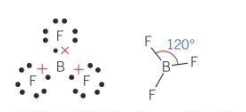
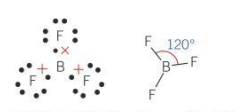
Bond angle for a planar molecule
120
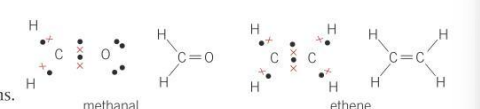
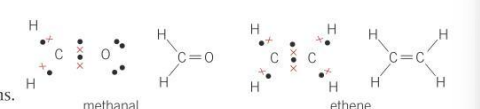
What kind of molecules are methanal and ethene?
planar
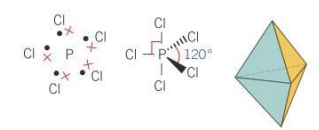
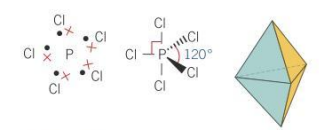
Bond angle for bipyramidal molecules
90 and 120
Bond angle for octahedral molecules
90
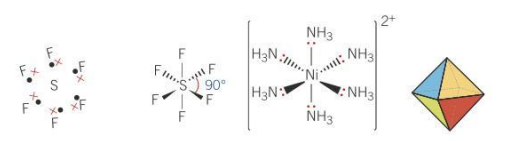
Electron pairs _____ each other as far away as possible but lone pairs repel ____ than bonded pair
repel
more
What is the shape of a molecules with 2 electron pairs with no lone pairs?
linear
What is the shape of a molecules with 3 electron pairs with no lone pairs?
trigonal planar
What is the shape of a molecules with 3 electron pairs with 1 lone pairs?
trigonal pyramidal
What is the shape of a molecules with 4 electron pairs with no lone pairs?
tetrahedral
What is the shape of a molecules with 4 electron pairs with 1 lone pairs?
trigonal pyramid
What is the shape of a molecules with 4 electron pairs with 2 lone pairs?
bent
What is the shape of a molecules with 5 electron pairs with no lone pairs?
trigonal bipyramidal
What is the shape of a molecules with 5 electron pairs with 1 lone pairs?
seesaw
What is the shape of a molecules with 5 electron pairs with 2 lone pairs?
T-shaped
What is the shape of a molecules with 5 electron pairs with 3 lone pairs?
linear
What is the shape of a molecules with 6 electron pairs with no lone pairs?
octahedral
What is the shape of a molecules with 6 electron pairs with 1 lone pairs?
square pyramidal
What is the shape of a molecules with 6 electron pairs with 2 lone pairs?
square planar
What is the shape of a molecules with 6 electron pairs with 3 lone pairs?
T-shaped
Bond angles of a seesaw
<120
<90
bond angle T-shaped
~90
Bond angle square pyramidal
<90
bond angle square planar
90