HYMS B11, W3
1/105
There's no tags or description
Looks like no tags are added yet.
Name | Mastery | Learn | Test | Matching | Spaced | Call with Kai |
|---|
No analytics yet
Send a link to your students to track their progress
106 Terms
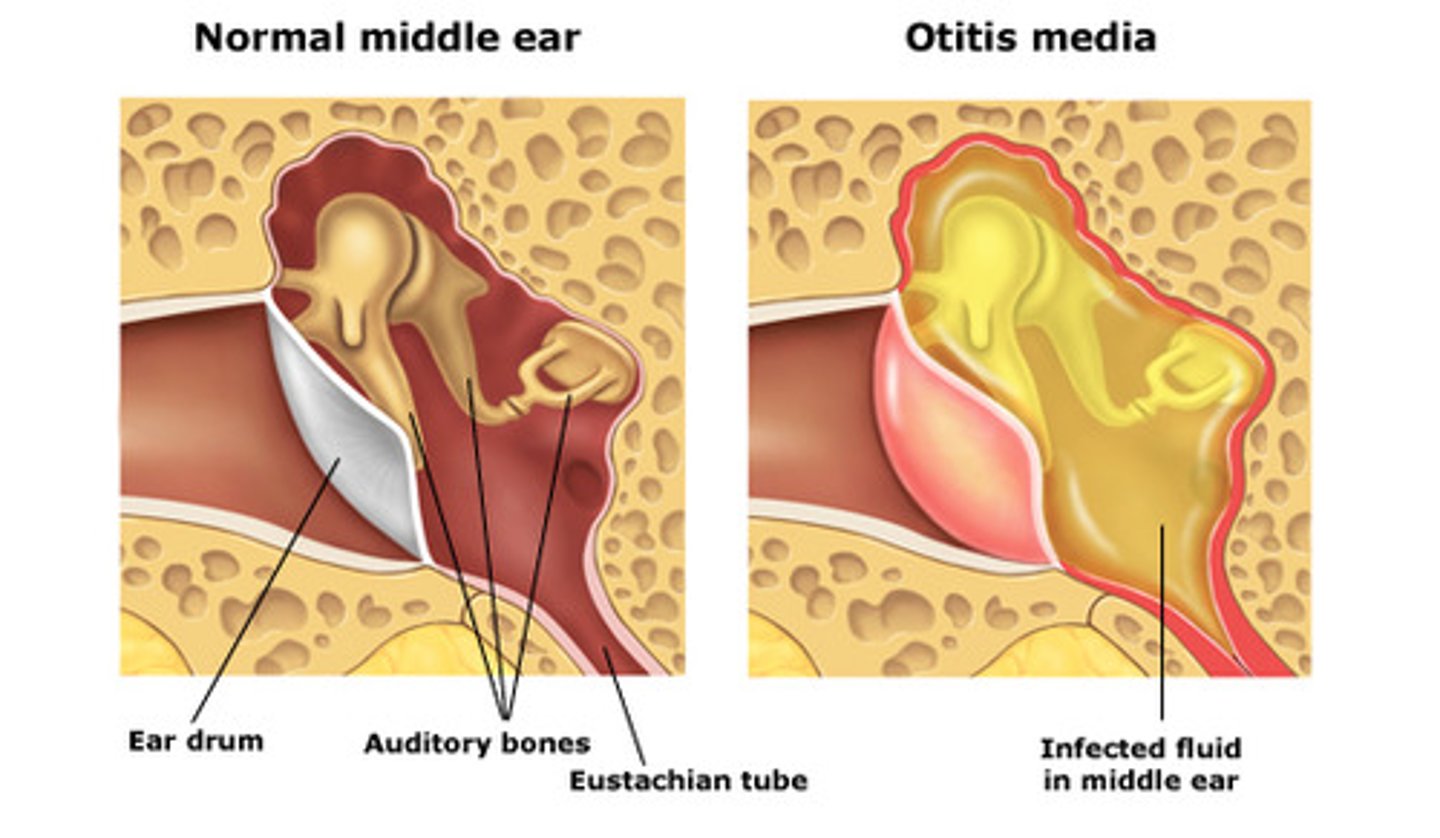
Otitis media
infection of the middle ear space, causing conductive hearing loss
common in children as they have small auditory / eustachian tube so can get blocked easily if they have a cold
types of hearing loss
conductive, sensorineural, mixed
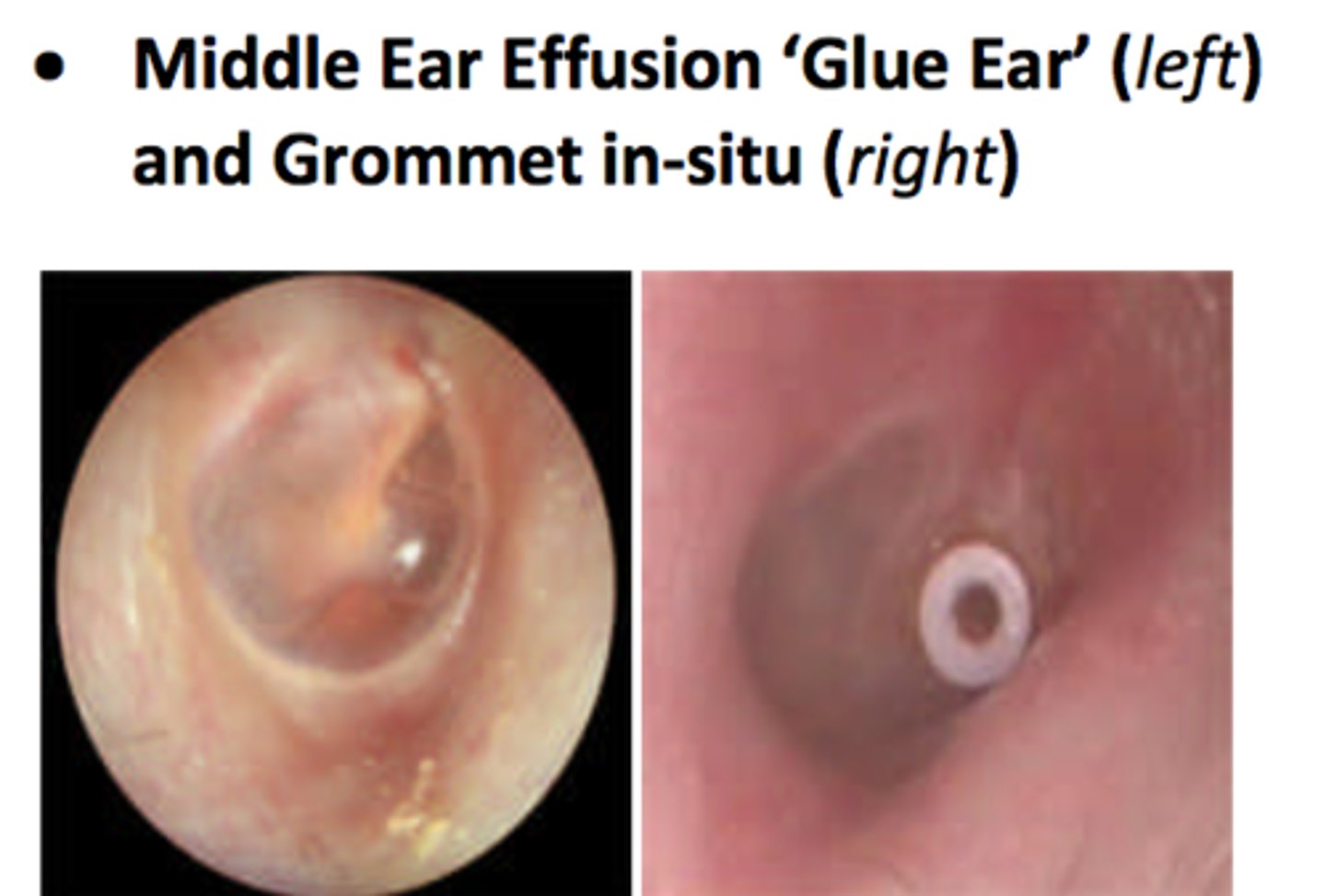
glue ear
a form of non-bacterial chronic otitis media; characterized by a persistent viscous effusion behind the ear drum
requires grommets if reoccurring or does not resolve
atelectasis
partial collapse or incomplete inflation of the lung.
significant vs never event
- never event - defined as Serious Incidents that are wholly preventable because guidance or safety recommendations that provide strong systemic protective barriers are available at a national level and should have been implemented by all healthcare providers.
- significant event - not as bad
what are some causes of birth defects?
- known genetic transition 20%
- chromosomal aberration 3-5%
- radiation <1%
- infection 2-3%
- maternal metabolic disorder 1-2%
- drugs + chemicals 4-6%
- unknown 65-70%
what is behavioural teratology?
effect on the 'behaviour or functional adaptation of the offspring to its environment' due to a tetragenic agent.
e.g, fetal alcohol syndrome
what is transplacental carcinogenicity?
type of reproductive toxicity where pregnant women is exposed to a teratogen. it causes no adverse effects on the mother but can cause cancer in the offspring. for example, diethylstilboestrol (DES).
what are some principles of teratogenic mechanisms?
- could be beneficial or harmless to mother but this does NOT mean it is to the embryo / foetus
- time of exposure is critical
- susceptibility to some teratogens is genetically determined
- teratogenic agents may be synergistic (worse in combination with others)
- a specific placental barrier doesn't exist
- risk may be altered by individual variation in drug - pharmacokinetic metabolism - hard to make blanket safe / unsafe rules
how has alcohol consumption in women of childbearing age and during pregnancy changed over recent years?
- total alcohol consumption has decreased
- BUT proportion of women binge drinking has increased
- patterns are likely to increase the risk of offsprings with prenatal alcohol exposure.
what are the two major types of inheritance?
- mendelian inheritance (monogenic) - shows as recognisable pattern
- multifactorial or polygenic - can show with a degree of sensitivity / more of a spectrum of presentation e.g. spina bifida
risk factors for spina bifida
- history of previous affected pregnancy with same partner
- inadequate folic acid intake
- pre-gestational diabetes
- valproate and carbamazepine
- maternal obesity
does a drug need to cross the placenta to affect the foetus?
NO
e.g. insulin
why does the pharmacokinetics of drugs alter in a pregnancy woman?
- maternal liver function / metabolism can alter throughout pregnancy
- increased renal blood flow and maternal excretion
- increased volume of distribution
- increased body water
- increased lung function
- lower GI motility
- lower albumin so higher conc. of unbound 'free' drug
how is data on teratogens obtained?
- epidemiological studies
- human case reports
- data is sacrce
-info is from preclinical studies in animals or in vitro studies
- issues with extrapolation
give some examples of congenital malformations
- anencephaly
- limb reduction
- transposition of the greater vessels
- cleft lip
- VSD
- syndactyly ('joined digits')
- hypospadias (opening of urethra is on the underside of the penis instead of the tip)
give some examples of drugs AND their potential fetotoxic effects during the first trimester
- androgens - virilisation of female fetus
- oestrogen's - feminisation of the male foetus
- warfarin - nasal hypoplasia, skeletal defects
- retinoids - craniofacial, CV, CNS
- diethylstilbestrol - uterine lesions, transplacental carcinogens
- anti epileptics - facial defects, mental retardation, neural tube defects
give some example of drugs and their potential fetotoxic effects after the first trimester.
- anti epileptics - possible mental retardation
- narcotics - neonatal respiratory distress
- warfarin - fetal haemorrhage, CNS abnormalities
- antidepressants - neonatal withdrawal symptoms
- BZs - floppy infant syndrome, neonatal resp. depression, withdrawal symptoms
- ACE inhibitors - oligohydraminous, growth retardation, lung + kidney hypoplasia, hypocalvaria, neonatal convulsions, hypotension, anuria
what are some risks to the foetus should be considered when prescribing?
- risk from the maternal illness
- risk from the treatment
- risk from failing to treat the mother
principles of prescribing in pregnancy
- only give drug when necessary - risk vs benefit
- use lowest effective dose for shortest time
- consider the stage of pregnancy
- avoid all drug treatment in first trimester if possible
- avoid all new drugs
- avoid poly pharmacy
what are some common conditions in pregnancy?
- pain
- nausea + vomitting
- constipation
- depression
- diabetes
- epilepsy
- haemorrhoids
- hay fever
- heartburn.+ dyspepsia
- hypertension
- thrush
- UTIs
- asthma
when are NSAIDs contraindicated in pregnancy?
after 20 weeks
non-pharmacological management of nausea and vomiting in pregnancy
1. small, high carb, low fat, frequent meals
2. ginger and/or acupressure
Pharmacological management of nausea and vomiting in pregnancy
1st. cyclizine, prochlorperazine,
2nd. metoclopramide
non-pharmacological management of constipation in pregnancy
1. increased fibre
2. increased fluids
3. increased exercise
what is thought to be the cause of constipation in pregnancy?
progesterone-induced intestinal smooth muscle relaxation
affects approx. 40% women
how does the presence of drugs in breast milk differ to levels in the mother's plasma?
- pH of breast milk is more acidic than that of plasma so basic compounds may reach higher concentrations in the milk
- Generally, drug binding to milk proteins is less than that to plasma proteins
- Most drugs are excreted in small amounts in breast milk
How can the risk of maternal drugs being passed to baby through breast milk be minimised?
- if the mother takes medicine immediately after breast feeding; avoids baby being exposed to peak maternal concentrations
- Delay breast feeding for 8h after taking drug e.g. senna
- Express and discard milk
These options are not appropriate if feeding frequently on demand
how common is post natal depression?
10-15 in every 100 women
what age are neonates?
birth to 1 month
what age are infants?
1 month to 1 year
what age are adolescents?
12-18 years
which factors need to be considered when prescribing in children?
ADME
- absorption - GI (affected by gastric acid secretions, bile salt formation, gastric emptying time, intestinal motility, microbial flora etc.); IV (dependant on muscle mass illness); transdermal, IM (thin skin); lungs (receptors less developed)
- distribution - increased total body water. higher volume of distribution so lower levels of plasma drug. higher doses of water soluble drugs needed / kg. decreased plasma proteins for drug binding. High bilirubin levels in neonates may displace drugs from albumin.
- metabolism - high metabolic rate (frequent dosing needed); less present or absent enzymes; some different metabolic pathways
- excretion - kidneys aren't fully matured till 6-7 months
how are doses calculated for children?
mostly based on body-surface area
Body-weight may be used to calculate doses expressed in mg/kg.
Young children may require a higher dose per Kg than adults because of their higher metabolic rates
how is maternal pCO2 and pO2 affected during pregnancy?
pCO2
- maternal pCO2 drops due to physiological hyperventilation
- produces an effect conc gradient for exchange
pO2
- maternal pO2 increases only marginally
- no diffusion gradient
- relative hypoxia in the foetus (but this works due to high affinity of HbF)
what are some adaptations that promote oxygen gas exchange in the foetus?
- HbF has a higher affinity for oxygen than HbA
- increased fetal haematocrit (more haemoglobin)
- increased maternal production of 2,3 DPG
- double Bohr and Haldane effects at the materno-fetal interface
fetal response to hypoxia
- HbF and increased Hb
- redistribution of flow to protect supply to heart and brain (reducing supply to GIT, kidneys and limbs)
- fetal HR slows to reduce O2 demand
- fetal chemoreceptors = vagal stimulation leading to bradycardia
- chronic hypoxaemia - growth restriction, behavioural changes and impact on development
what are the three shunts present in the fetal circulation, not found in adult circulation?
1. ductus venous
2. foramen ovale
3. ductus arteriosus
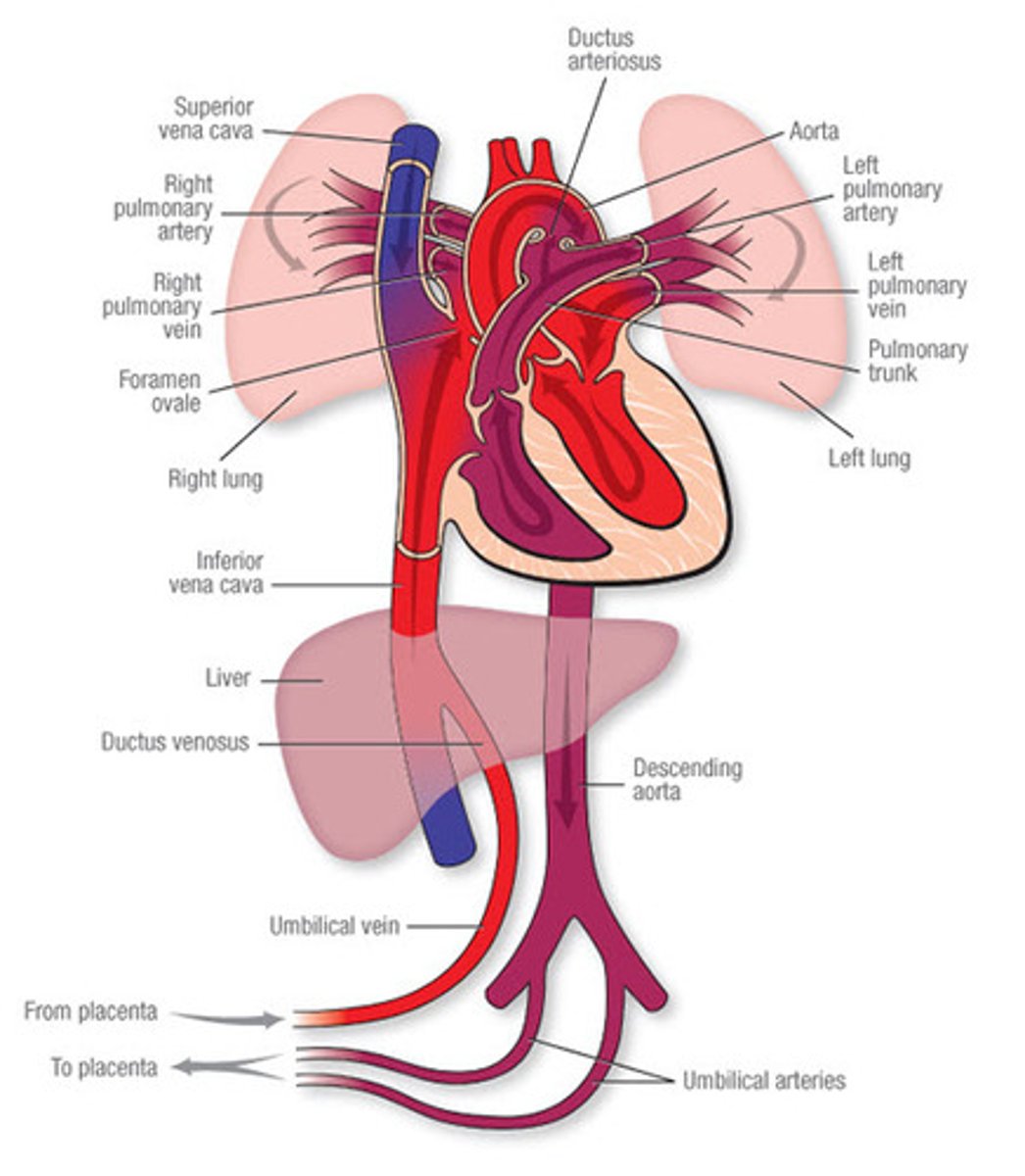
role of the ductus venosus
- connects the umbilical vein (oxygenated blood to the IVF)
- blood enters right atrium
- by bypassing blood around the liver, O2 saturation is mostly maintained
role of the foramen ovale
- RA pressure is greater than LA pressure so FO is open
- free border of the septum secundum creates a crest which allows for two streams of blood flow
- most flows to the LA, some flows to RV mixing with oxygenated blood from SVC
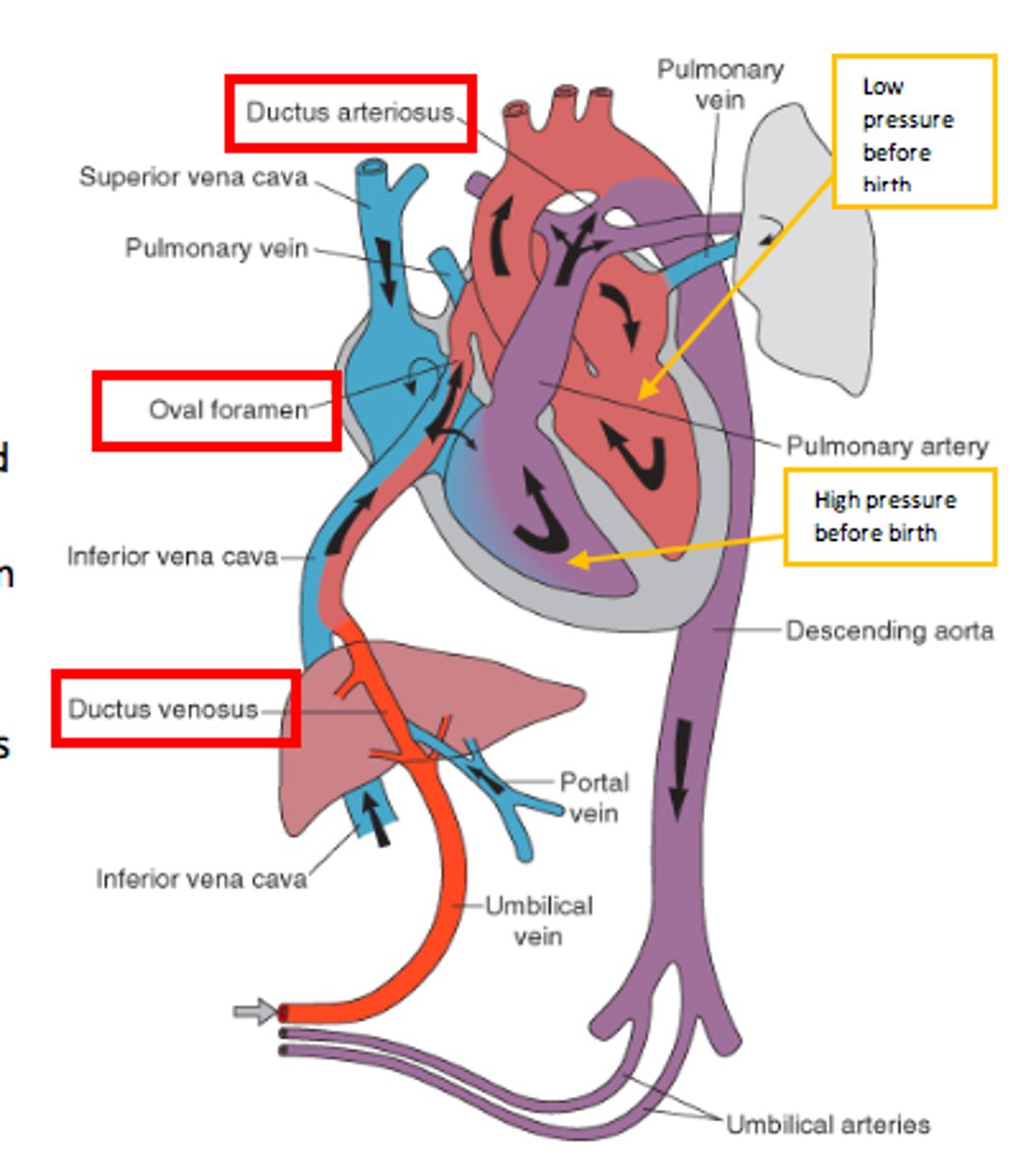
role of the ductus arteriosus
- shunts blood from RV and pulmonary trunk to the aorta
- joins the aorta distal to the supply to the head (and heart)
- this minimises the drop in O2 sats.
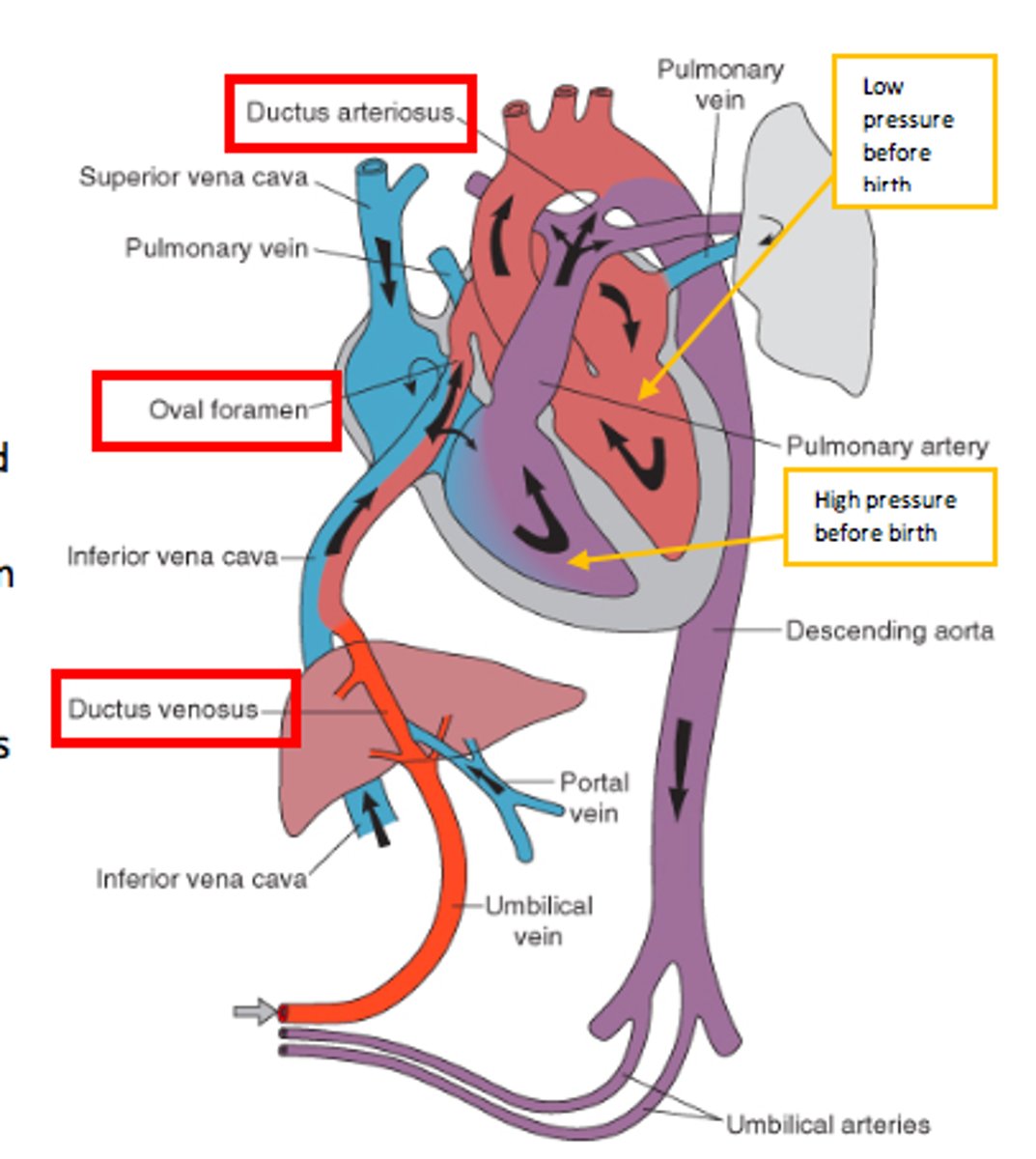
how does the foramen ovale close?
- Baby takes breath, and gets rid of fluid and take in air.
- Pressure on RA drops and LA increases.
- FO closes due to increased L atrial pressure
how does the ductus arteriosus close?
1. Physiological at first - spasm of vascular smooth muscle in the ductus in response to increased pO2
2. Anatomical - transformation to a fibrous cord
how does the ductus venosus close?
when the umbilical cord is closed, flow in the DV stops due to removal of placental support
becomes the ligamentum venosum
respiratory distress syndrome (RDS)
A respiratory disorder that affects premature infants born without enough surfactant in the lungs. It is treated with respiratory support and surfactant administration
what is a congenital deformation?
late changes in previously normal structures (mechanical defect due to aberrant force that distorts the structure).
'was okay, got squashed'
what is a congenital disruption?
a secondary disturbance due to early influence of external factors. it is non-progressive and due to the breakdown of a body structure that had a normal developmental potential.
'starts out ok then goes wrong'
what is a congenital malformation?
primary disturbance of embryogenesis. it is a non-progressive congenital morphological anomaly of a single organ or body part.
blue print is wrong so it is made wrong
primary vs secondary congenital anomaly
primary
- at the genetic level. single gene, chromosomal or polygenic
secondary
- an external factor e.g. teratogen
note - significant proportion of anomalies have unknown aetiology
polytopic field defect
pattern of defects derived from the disturbance of a single developmental field
anomaly/insult at 2-4 weeks
defect will go through to all the structures yet to develop = scattered
monotopic field defect
A localised pattern of defects that occurs as part of later effects in weeks 4 to 8
e.g. only upper limb defect
fetal period anomaly
insult from >9 weeks
organogenesis so organs affected
reduced risk of big fetal structural anomalies except from the brain and CNS which is continuing to develop (so still susceptible)
what is a sequence congenital defect?
- one initial malformation/disruption/dysplasia/deformation from an insult
- creates other late secondary effects (like dominos)
pre-embryonic fetal defect consequences
- occurs within the first two weeks
- tends to be lethal
examples of congenital deformation
- talipes
- congenital hip dislocation
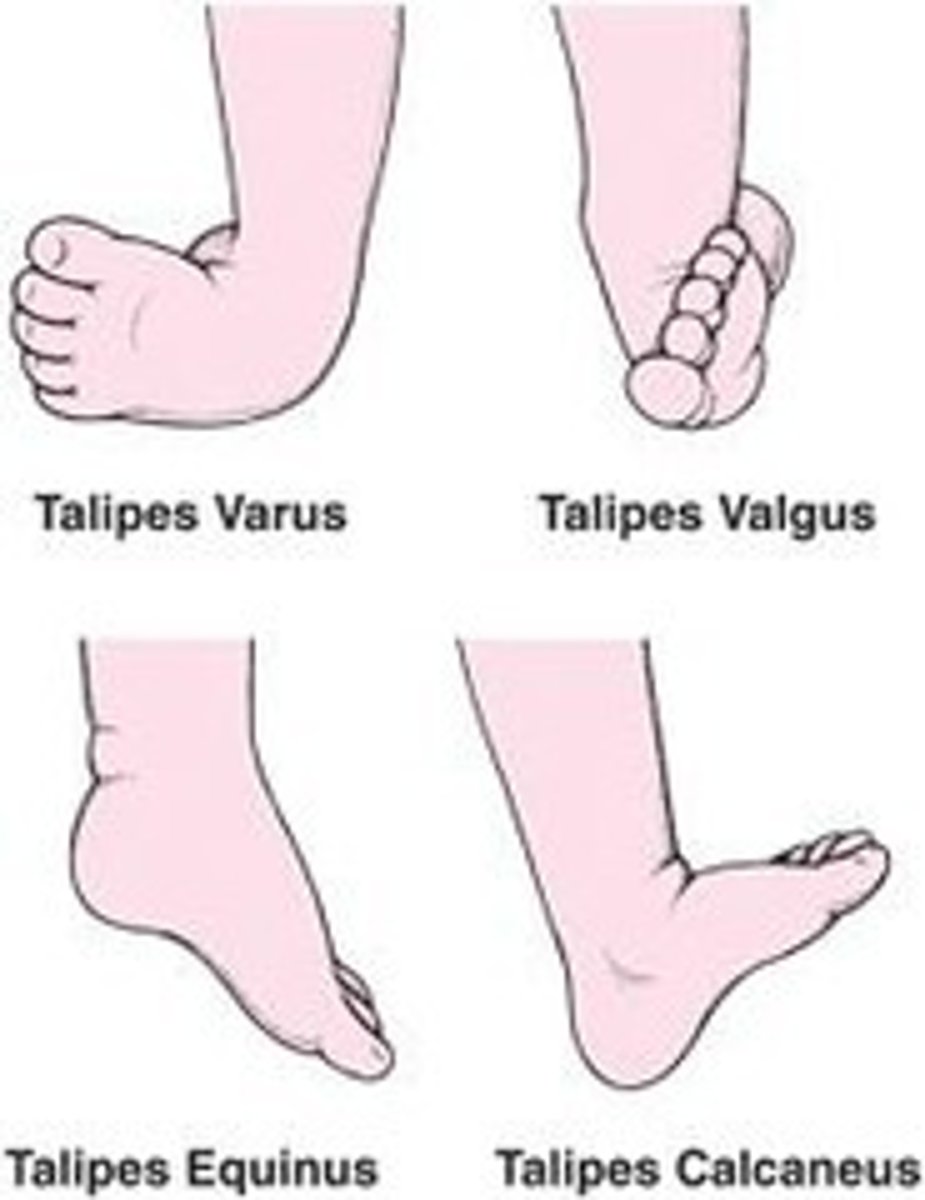
examples of congenital disruption
- amniotic bands - strips of amniotic membrane can wrap around structures e.g. limbs / fingers and cut off blood supply
- poland anomaly - interruption/occulsion of subclavian artery stopping blood supply to pectoralis major so muscle doesn't develop (unilateral effect)
example of congenital sequence
- In Potters sequence where facial appearance, lung hypoplasia, and joint contractures are all the result of constraint due to insufficient amniotic fluid (due to renal anomalies or urethral valves).
- Pierre robin sequence - mechanism theory. begins with mandibular hypoplasia. tongue can not drop as a result.
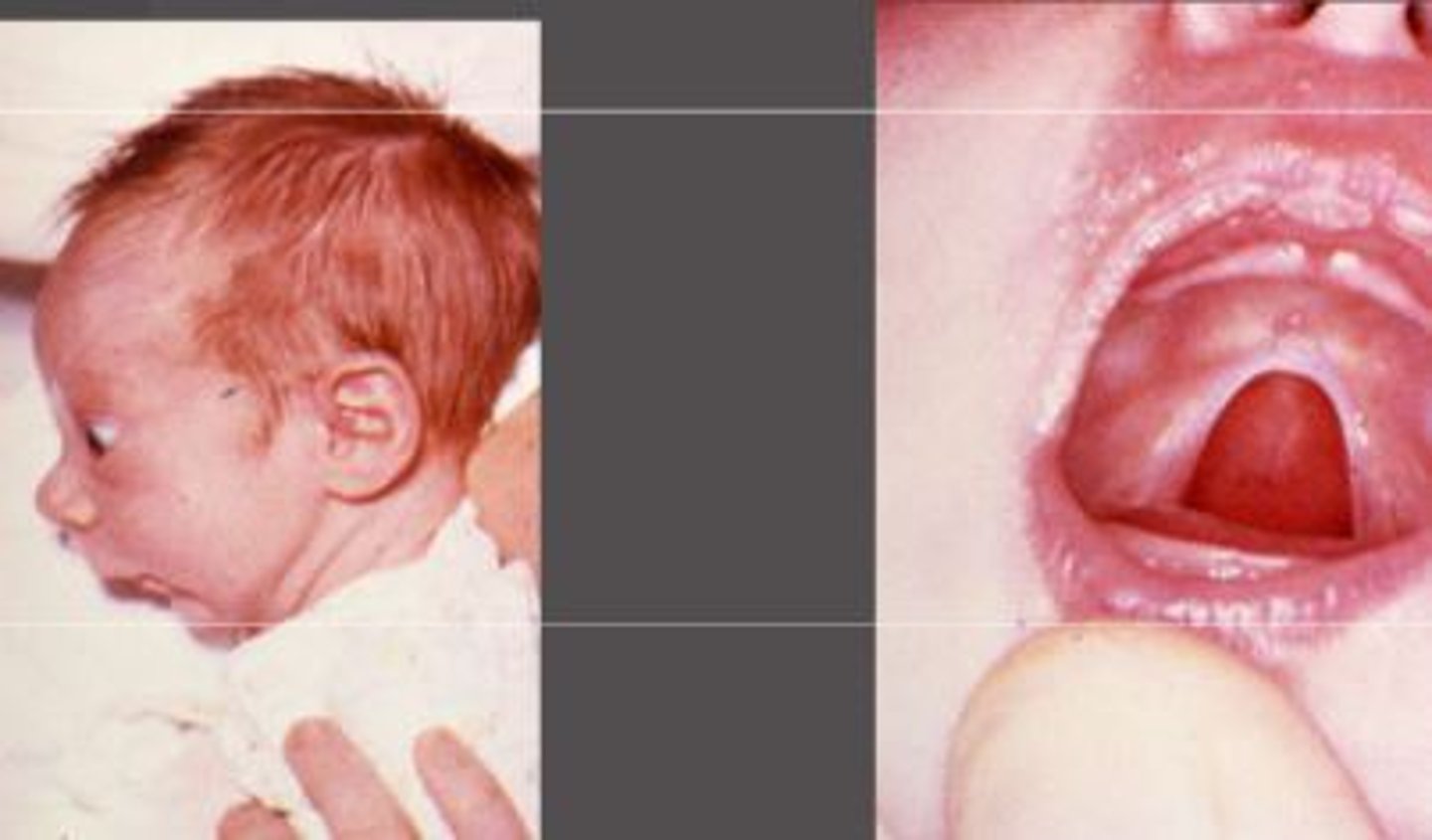
DI george syndrome
- deletion of section of chromosome 22 (22q11.2)
- failure of development of 3rd and 4th pharyngeal pouches
- and therefore anomalies to all deriving structures
CHARGE syndrome
Coloboma
Heart defects
A choanal atresia
R growth and developmental retardation
Genital hypoplasia
Ear defects
CHD7 heterozygous mutation. CHD7 expression is essential for the production of multipotent neural crest cells.
meningocele
the congenital herniation of the meninges through a defect in the skull or spinal column
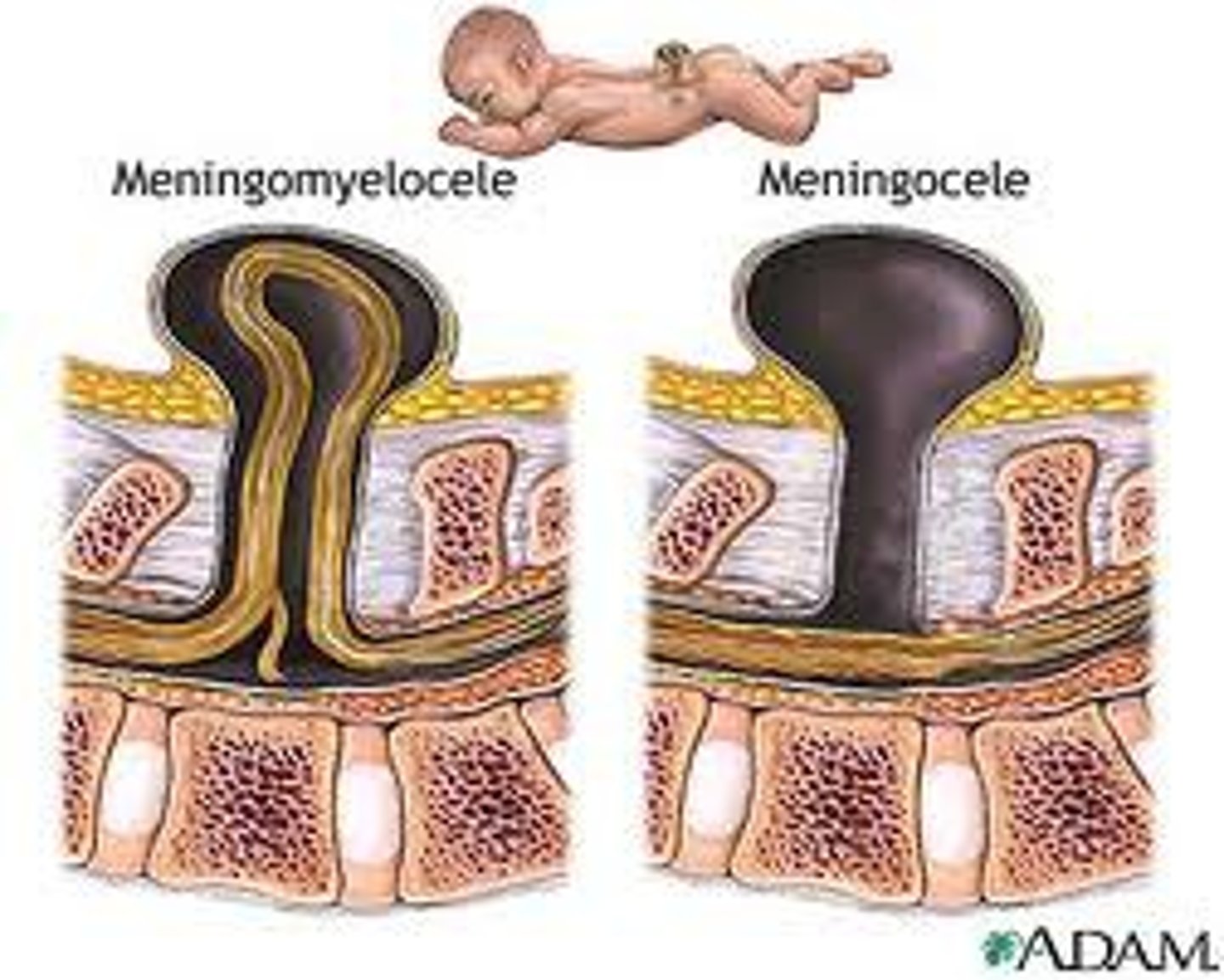
myelomeningocele
most severe form of spina bifida in which the spinal cord and meninges protrude through the spine
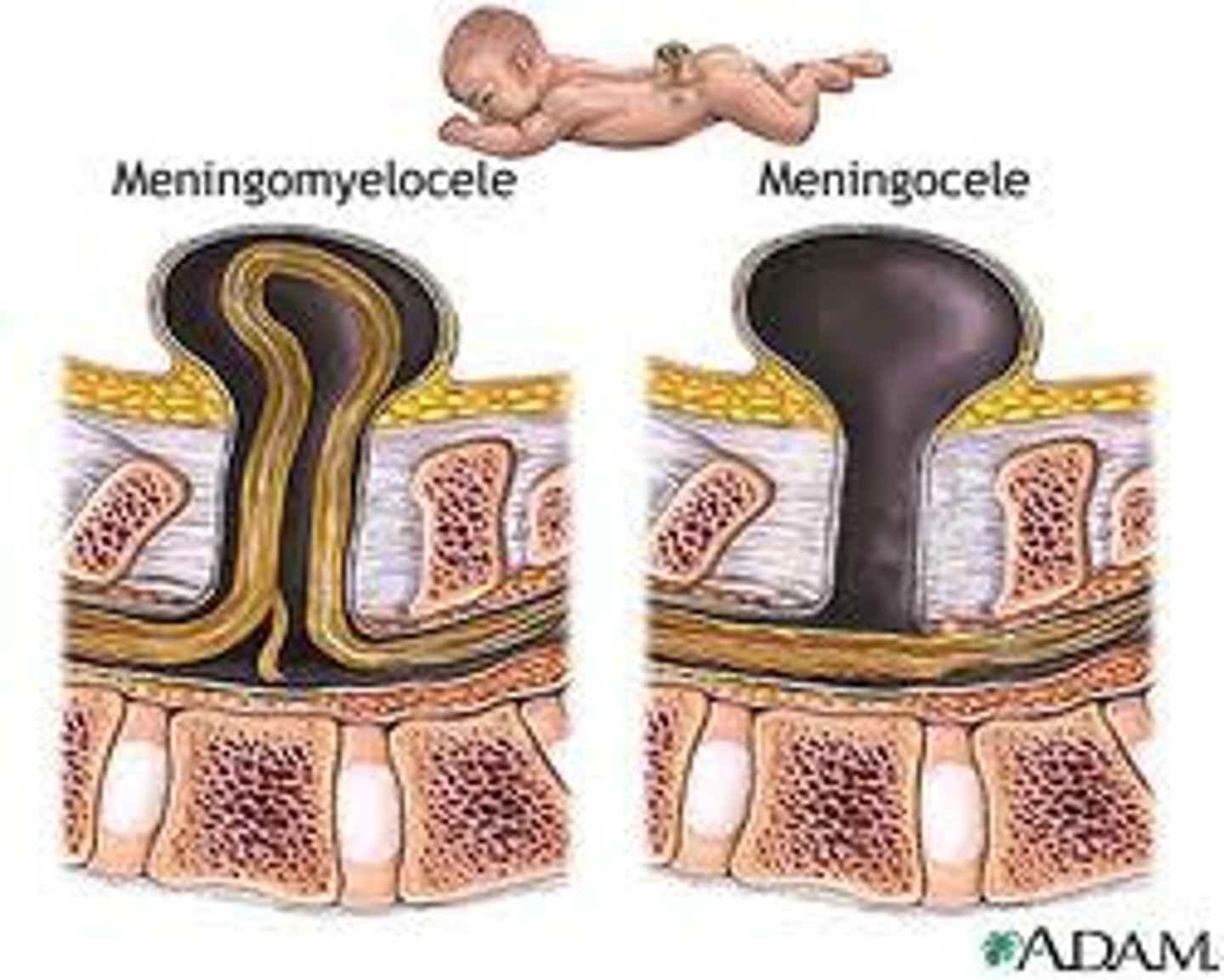
explain why the facial skeleton is affected in babies with fetal alcohol syndrome
- facial skeleton is derived from neural crest cells populating the pharyngeal arches
- neural crest migration and development of the brain are extremely sensitivity to alcohol
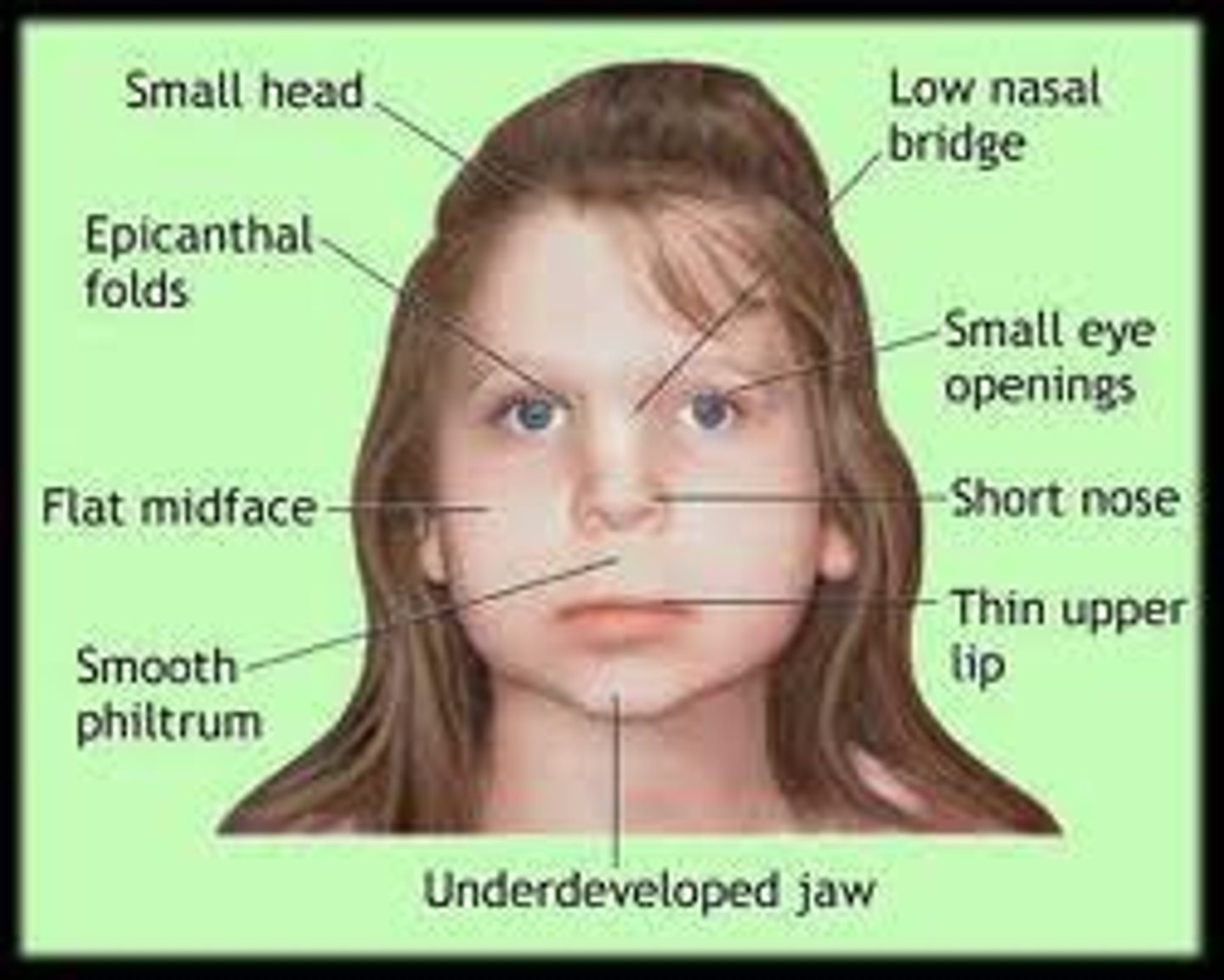
congenital rubella syndrome
Classic triad:
1. congenital cataracts (white pupils)
2. sensory-neural deafness
3. Patent ductus arteriosus (could be pulmonary artery stenosis as well)
Could potentially also result in microcephaly and mental retardation
non-pregnant females should be vaccinated with the live MMR vaccine prior to pregnancy.
rubella is a teratogent
how common are congenital anomalies?
affect 3% of live born infants
describe how screening can prevent congenital anomalies
- pre-conception genetic counselling
- antenatal screening - first trimester offered triple test and nuchal fold measurement. second trimester anomaly scan at 20 weeks
- newborn screening examination
Outline the impact of congenital defects on the child, family and society.
- affects 3% liveborn infants. causes perinatal and neonatal death and disability
- lifelong impact to child and family. 20-30% of admission to tertiary care are due to congenital anomaly. emotion and physical wellbeing toll as well as personal financial cost.
- historical beliefs regarding causation e.g. witchcraft/supernatural beliefs are still present in some cultures. Important to be able to explain this.
what are the four main areas of development?
- gross motor
- fine motor and vision
- hearing, speech and language
- social skills and behaviour
what are the language developmental milestones?
2months - Co
4months - Haha
6months - Babble
9months - Mommy Dada
12months - 1+ word
look at the no. letters in each milestone
what are the gross motor developmental milestones?
2months - Raise head
4month - role over
6months - sits up
9months - crawl
12months - walk
sing to tune of happy birthday:
"2 months old raise your head,
4 months old roll over fred
6 months sit up, 9 months crawl,
12 months walk, and that's all!"
what are the social developmental milestones?
2 months - Twinkle (smiles)
4 months - Focuses on sound
6 months - Stranger anxiety
9 months - Name (responds)
12 months - Told commands (responds)
First letter in each milestone = first letter of the no. of month
how is development assessed?
- history from parents + other caregivers (medical and social etc as per normal history)
- observe the child play and in formal assessments
- consider ability to perform as well as quality of performance
- compare to normal ranges
at what age will 95% of children (2SD) be walking?
18 months
the remaining 5% are mostly likely to be normal late walkers. small risk of neurological / muscular disease.
what are some reasons children may not meet their hearing, speech or language developmental milestones?
- hearing input issues (conductive or sensorineural loss)
- forming the words (muscles and palate issues - mechanical)
- content of speech (speech area in brain and connections)
- part of global developmental delay
- environmental deprivation
- Autism spectrum disorders
what are some psychological needs for development?
- security
- role models
- attention
- play
- opportunity to learn from experience
- self respect
- independence
- personal identity
what are some developmental red flags?
- regression
- not fixing and following
- not responding to noise
- abnormal tone
- no smile at 8 weeks
- not holding objects at 5 months
- early hand preference
- not sitting at 12 months
- not walking at 18 months
- persistent toe walking
- not pointing at objects at 2 yrs
list some causes of developmental problems
- genetic syndromes e.g. trisomy 21
- congenital infections (TORCH)
- cerebral malformation +/- hydrocephalus
- antenatal insults
- perinatal hypoxia / hypoglycaemia
- postnatal meningitis / trauma / metabolic insults
- deprivation and / or abuse
what influences growth?
- genetic potential
- optimal intra-uterine conditions (IUGR)
- optimal post natal nutrition
- normal hormonal status
- good health
- good diet
- nurturing social environment
what is failure to thrive (FTT)?
- suboptimal weight gain in infants and toddlers
- most will lie below the second gentile
- it is a description not a diagnosis
- mainly weight - watch height and head circumference
- first sign is failing to meet growth expectations on chart
- weight must fall across the centiles
- trajectory is very important - need multiple measurements
classifying of FTT
- organic. due to an acute or chronic medical condition that interferes with normal food intake, absorption or digestion of food, or is due to increased calorie need to keep up or help growth.
- non-organic (majority). this includes a broad spectrum of psychosocial, environmental and /or economic deprivation
- undernutrition occurs in both
causes of FTT
- inadequate intake - poor technique, wrong milk, social issues
- inadequate retention - vomiting, GORD
- malabsorption - CMPI, CF, short gut
- failure to utilise nutrients - renal/liver disease, IEMs
- increased requirements - thryotoxicosis, CF, chronic infections, malignancy, heart disease
what is used instead of BMI for children?
RCPCH growth chart
potential causes of short stature in children
- mostly genetic / constitutional
- growth hormone deficiency
- panhypopituitarism
- hypothyroidism
- steroid excess
- skeletal dysplasias
- chromosomal disorders
give some examples of fine motor and vision skills in development
Grabbing objects
Transferring hand to hand
Building blocks
Radial-palmer grasp
Mature pincer grasp
Scribbling and shapes
what are the key ages when considering weight changes in children?
wk 1 - lose up to 10% birth weight
wk 2 - regain birth weight
5 months - weight doubled
1 yr - weight tripled
how can nutrition in children be assessed?
- weight and height
- mid upper arm circumference - muscle mass
- skin fold thickness - subcut fat stores
- low albumin
- low vit and minerals
- nbc
- dietary recall and diary
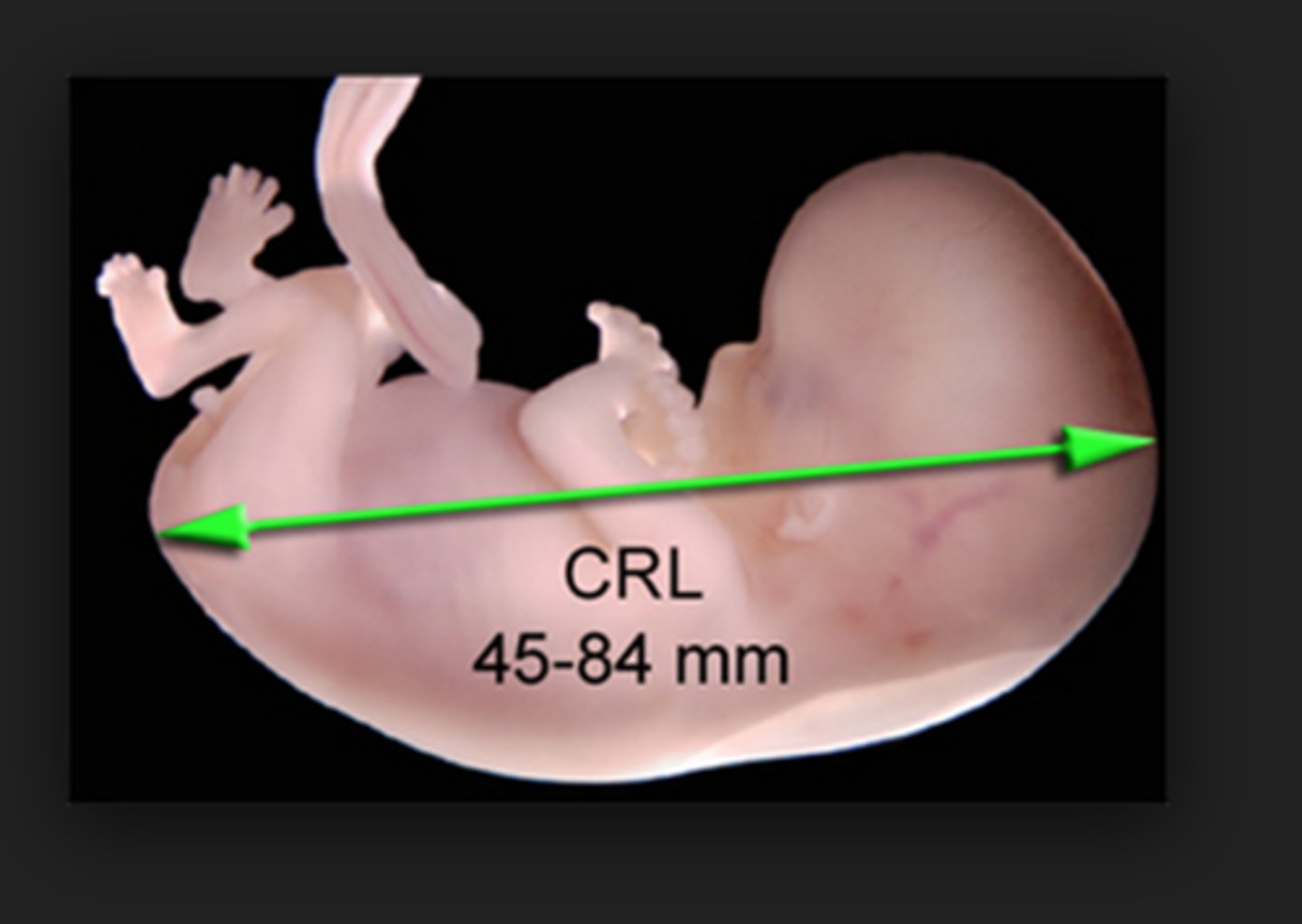
crown-rump length (CRL)
- most accurate measurement of the embryo in the first trimester
- used to date pregnancy and estimate due date
used between 7-13 weeks
- also used to check location, number and viability
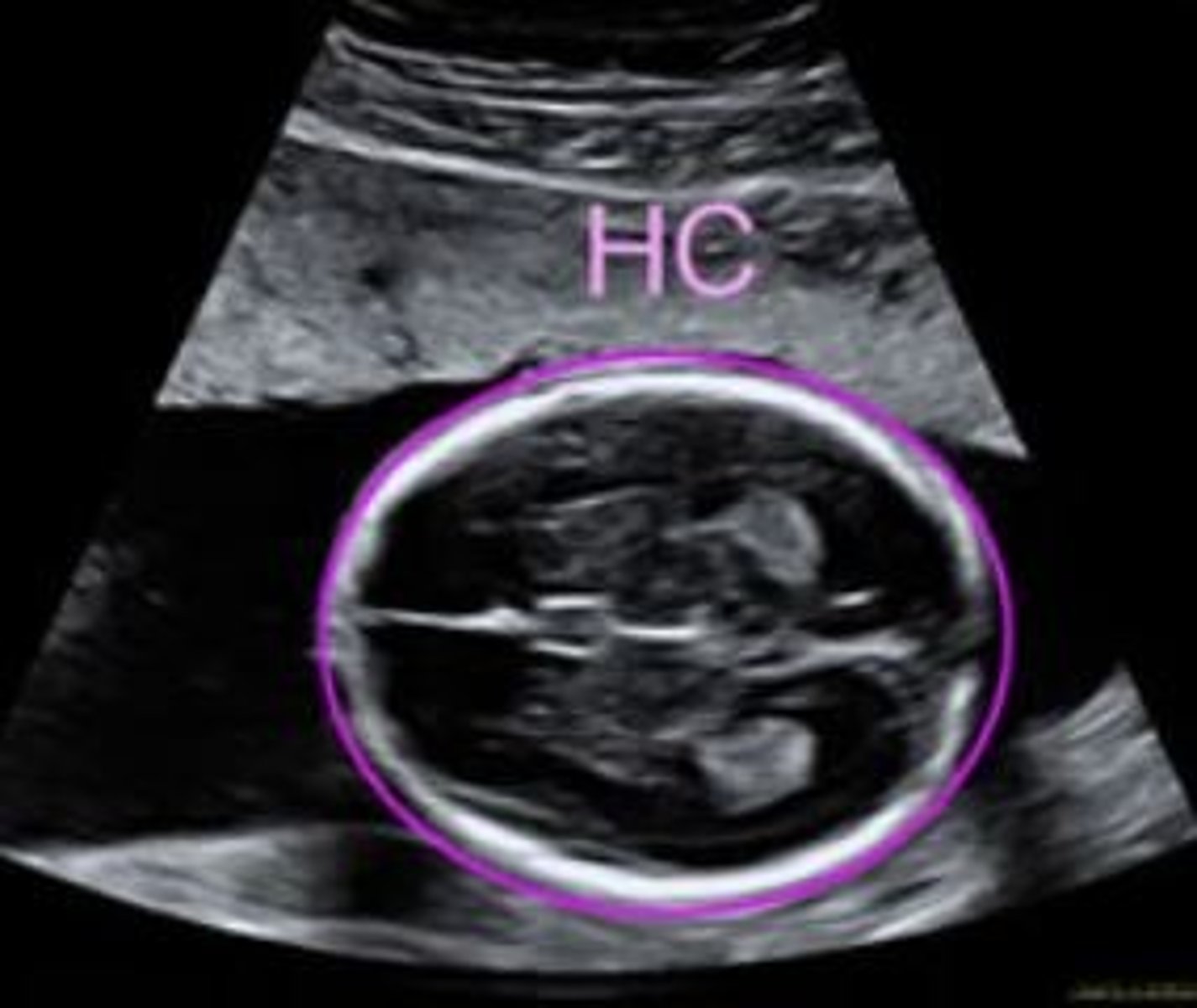
name three measurements taken of the foetus in utero
- Head circumference
- Abdominal circumference
- Femur length
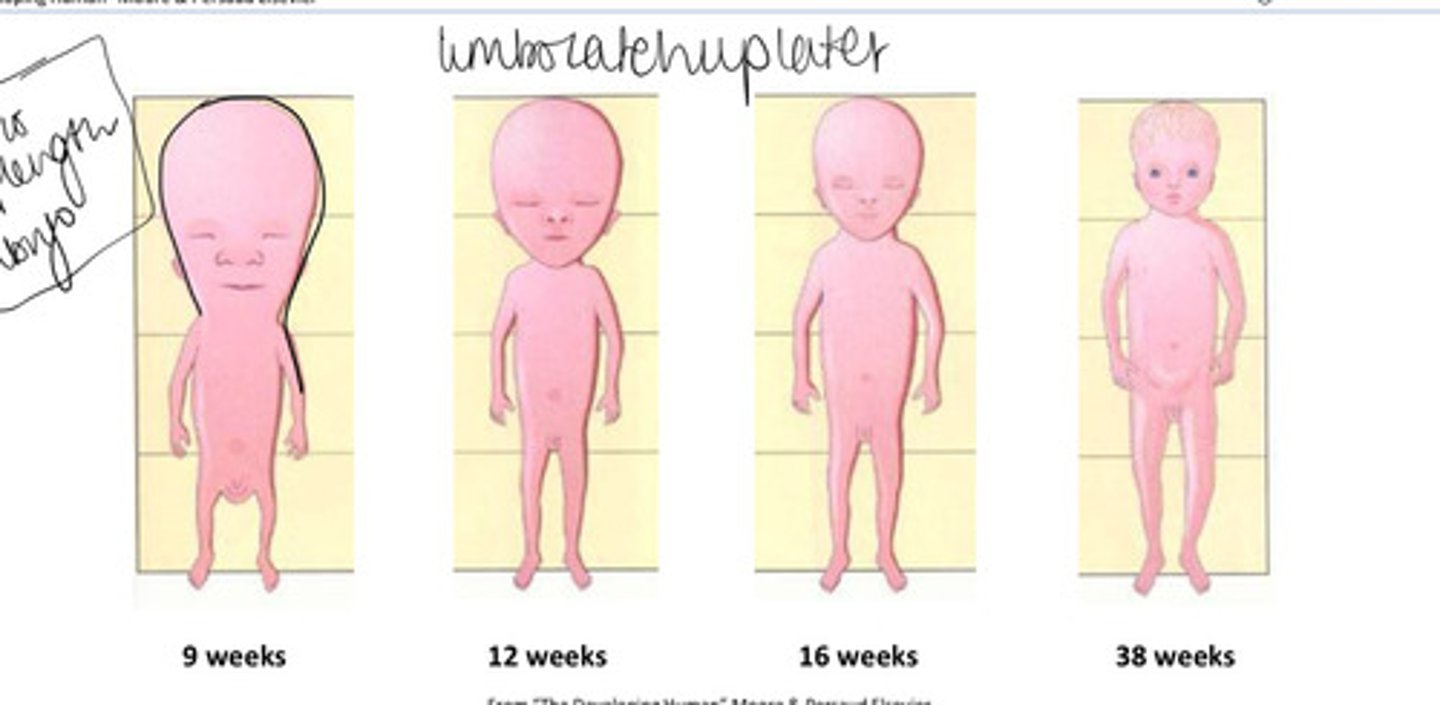
how does body proportions of the foetus change during pregnancy?
9 weeks the head is around half of the CRL
this lessens from 12 wks as body length and lower limb growth accelerates
what is the dominant cellular growth mechanism throughout pregnancy?
0-20 - hyperplasia
20-28 - hyperplasia + hypertrophy
28-term - hypertrophy
what is considered an average birth weight?
3500g
= 7.7 lbs
what is considered the upper ends of small and large birth weight?
<2500g / 5.5lbs suggests growth restriction
>4500g / 9.9lbs is macrosomia (indicates maternal diabetes)
what are some influences of birth weight ?
- placental
- fetal (low Papp-A echogenic bowel)
- maternal (smoking, BMI extremes, hypertension)
- may just be constitutionally small
what are some antenatal methods of assessing fetal growth and well being?
- mother - fetal movements
- biochemical tests
- regular measurements of uterine expansion (symphysis-fundal height)
- ultrasound scan
evaluate biochemical tests for fetal wellbeing
- expensive
- poor PPV
evaluate symphysis-fundal height as an assessment of fetal growth
- relatively unreliable
- non-invasive
- relatively cheap
- allows identifying for need of further assessment
evaluate the use of ultrasound as an assessment of fetal growth
- safe
- can be used in early pregnancy to calculate age, rule out ectopic and number of fetuses etc
- routinely carried out at 20 weeks to assess fetal growth and anomalies.
evaluate the use of dopplers in assessing fetal health
- use alone in low risk or unselected populations does not improve maternal or neonatal outcomes
- used in diagnosis not screening
- change in waveform allows for further surveillance
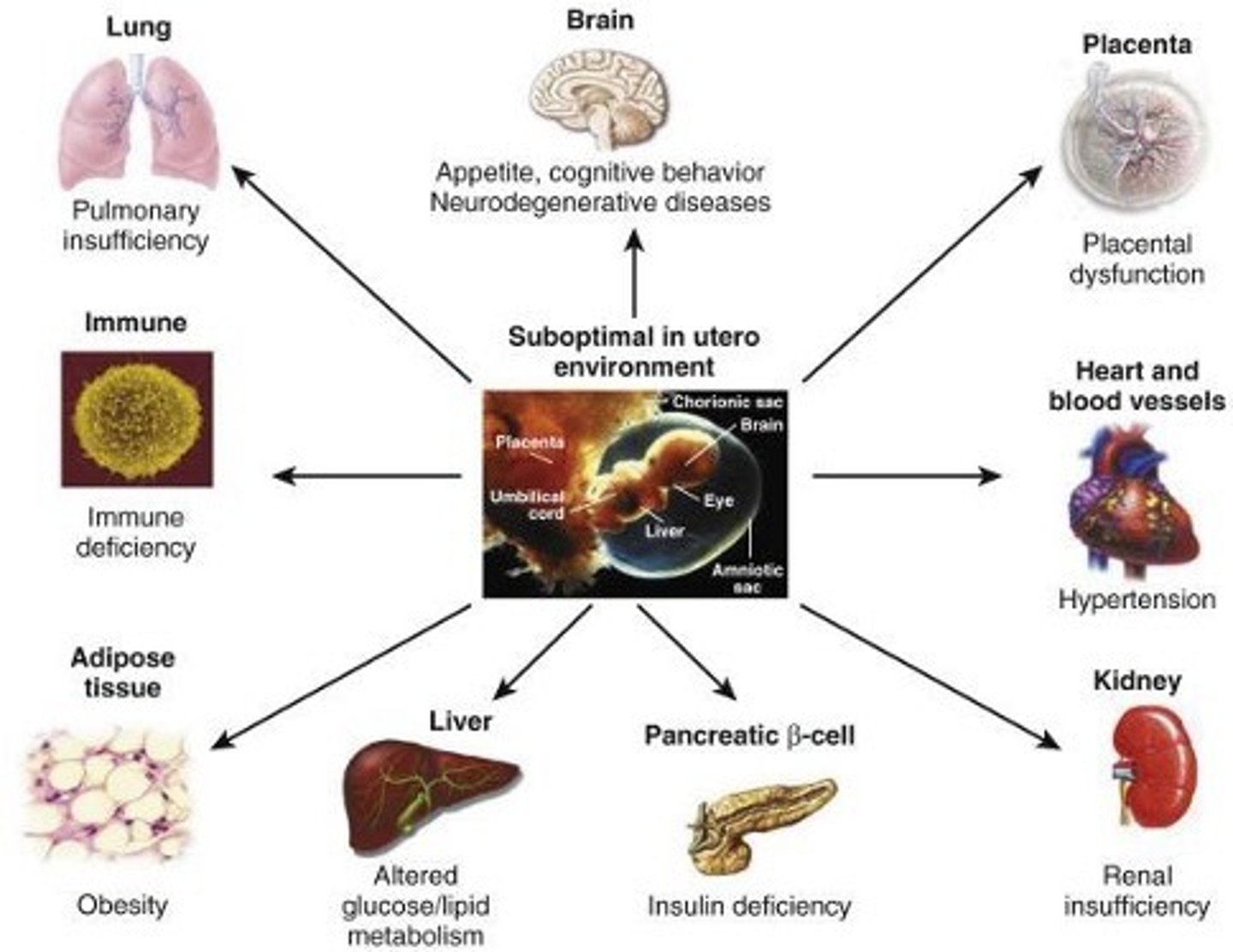
Describe long term consequences of fetal growth restriction.
- barker hypothesis
- fetal programming leading to adult metabolic syndrome
causes of death in DMD
respiratory muscle weakness
cardiomyopathy
death usually occurs in 20s
etiology of Duchenne muscular dystrophy (DMD)
- X-linked recessive
- on chromosome Xp21
- causes a mutation in the dystrophin gene