Chemistry Ch 5 Chemical Bonding and Molecular Geometry
1/16
Earn XP
Description and Tags
Name | Mastery | Learn | Test | Matching | Spaced |
|---|
No study sessions yet.
17 Terms
Valence Electron
Electrons on the outermost energy level of an atom that are able to bond and/or react
Ionic Bond
Transfer of electrons between a Metal and Nonmetal.
Metal: low electronegativity (gp 1&2)
Non Metal: high electronegativity (gp 16,17)
Covalent Bond
A sharing of electrons between a Nonmetal and a Nonmetal, or a Nonmetal and itself.
Metallic Bond
A common exchange of delocalized electrons between a Metal and a Metal, or a Metal and itself
Delocalized Electrons
Electrons that are not stationary, and are free to move between atoms in a bond.
Octet Rule
All atoms want to obtain the electron configuration of the nearest Noble Gas, meaning that they want to have 8 valence electrons in their outermost energy shell.
All NG have 8 outer shell electrons, except for He which has 2
Lewis Dot Diagrams
Each dot represents an element's valence electrons. "Dots" are placed around the element (in any direction, but one dot/e- on each side before pairing up) to represent the val e-.
1. Work out the Noble Gas Configuration of the element
2. Find the outermost energy level, and highlight/circle those e-
3. The number of e- in the outermost level are the dots to be placed around the element in the drawing.
Check: the LAST number in an element's group # is the number of val e-.
Ex) C
[He] 2s2, 2p2
2 is the largest energy level. 2 e- + 2e- = 4e-
Check: C is in group 14, so it will have 4 val e-
Note: Although He is in gp 18, it only has 2 val e- and they are paired together b/c it is a Noble Gas (does not react)
![<p>Each dot represents an element's valence electrons. "Dots" are placed around the element (in any direction, but one dot/e- on each side before pairing up) to represent the val e-.</p><p>1. Work out the Noble Gas Configuration of the element</p><p>2. Find the outermost energy level, and highlight/circle those e-</p><p>3. The number of e- in the outermost level are the dots to be placed around the element in the drawing.</p><p>Check: the LAST number in an element's group # is the number of val e-.</p><p>Ex) C</p><p>[He] 2s2, 2p2</p><p>2 is the largest energy level. 2 e- + 2e- = 4e-</p><p>Check: C is in group 14, so it will have 4 val e-</p><p>Note: Although He is in gp 18, it only has 2 val e- and they are paired together b/c it is a Noble Gas (does not react)</p>](https://knowt-user-attachments.s3.amazonaws.com/f03e29ab-7ce3-475b-bb78-5e9d556e9eaf.jpg)
Ionic Bonding Terms:
Halide
Salt
Crystal Lattice
Halide: a salt that contains a halogen. Ex) KBr
Salt: any ionic compound. Ex) NaCl
Crystal Lattice: a repetitive arrangement of atoms in an ionic compound
Properties of Ionic Compounds
1. High melting point
- the stronger the attraction is btwn ions, the higher the MP will be.
2. Do not conduct electricity when solid form
- as a solid, the ions are bound up in the lattice (no free moving ions) so they do not conduct
3. Solid at room temperature
4. Good conductors when dissolved or in liquid form
- the water/heat breaks the lattice, allowing for free moving ions. This leads to high conductivity
5. Brittle and Hard
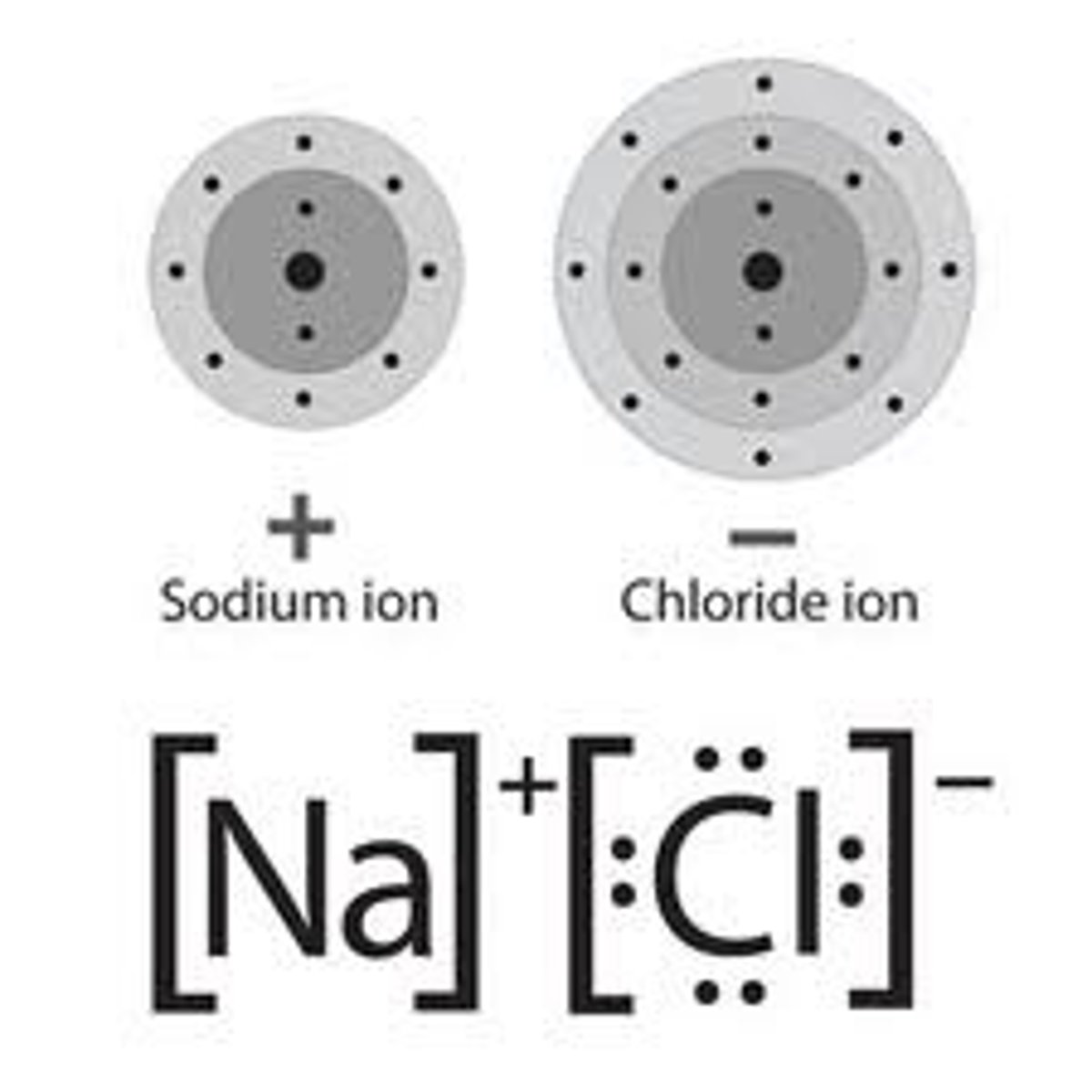
Ionic Bonding Diagrams/How To Draw
1. Begin with the cation (+/first element in cmpd). Draw its Lewis Dot Diagram with proper val e-.
2. Then, draw the anio (-/second in cmpd) with the val e-.
3. The cation will give its val e- to the anion, so draw an arrow from its val e- to the "open space" of the anion.
4. Repeat until the cation no longer has val e-, and the anion is full. You may need to draw more than one of an element depending on the number of val e-.
5. Then, draw the bond. Put the cation first, draw the LDD, its charge above/superscript (from your PT) and the number of atoms below (subscript) if more than one were needed
6. Now, the anion. Put it in brackets, and draw the LDD, indicating where e- were added. Write its charge in the superscript, and number of atoms in the subscript.
Covalent Bonding Terms
Electronegativity
Nonpolar Cov Bond
Polar Cov Bond
Dipole
Electronegativity: the ability of an atom, when in a bond, to attract an electron pair
Nonpolar Cov Bond: an EQUAL sharing of electrons btwn atoms in a bond
Polar Cov Bond: an UNEQUAL sharing of electrons btwn atoms in a bond
Dipole: a molecule with unequal electron distribution, creating partial positive/negative charges
Types of Cov Bonds
Single
Double
Triple
Single: one pair of e- shared btwn atoms in a bond. Ex) H-H
Double: two pairs of e- shared btwn atoms in a bond Ex) O=O
Triple: three pairs of e- shared btwn atoms in a bond. Ex) N=-N
Using EN values to figure out bond types
Using Ketelaars Triangle, we can figure out the type (I, PC, NPC).
Subtract the larger EN value (of the two elements in a bond) from the smaller. This will be the Y axis value.
Find the average EN value by adding them up and dividing by 2. This is the X axis value.
Find where the two values intersect. This will tell you what type the bond is.
Note: "Covalent" at the bottom means Non Polar Cov.
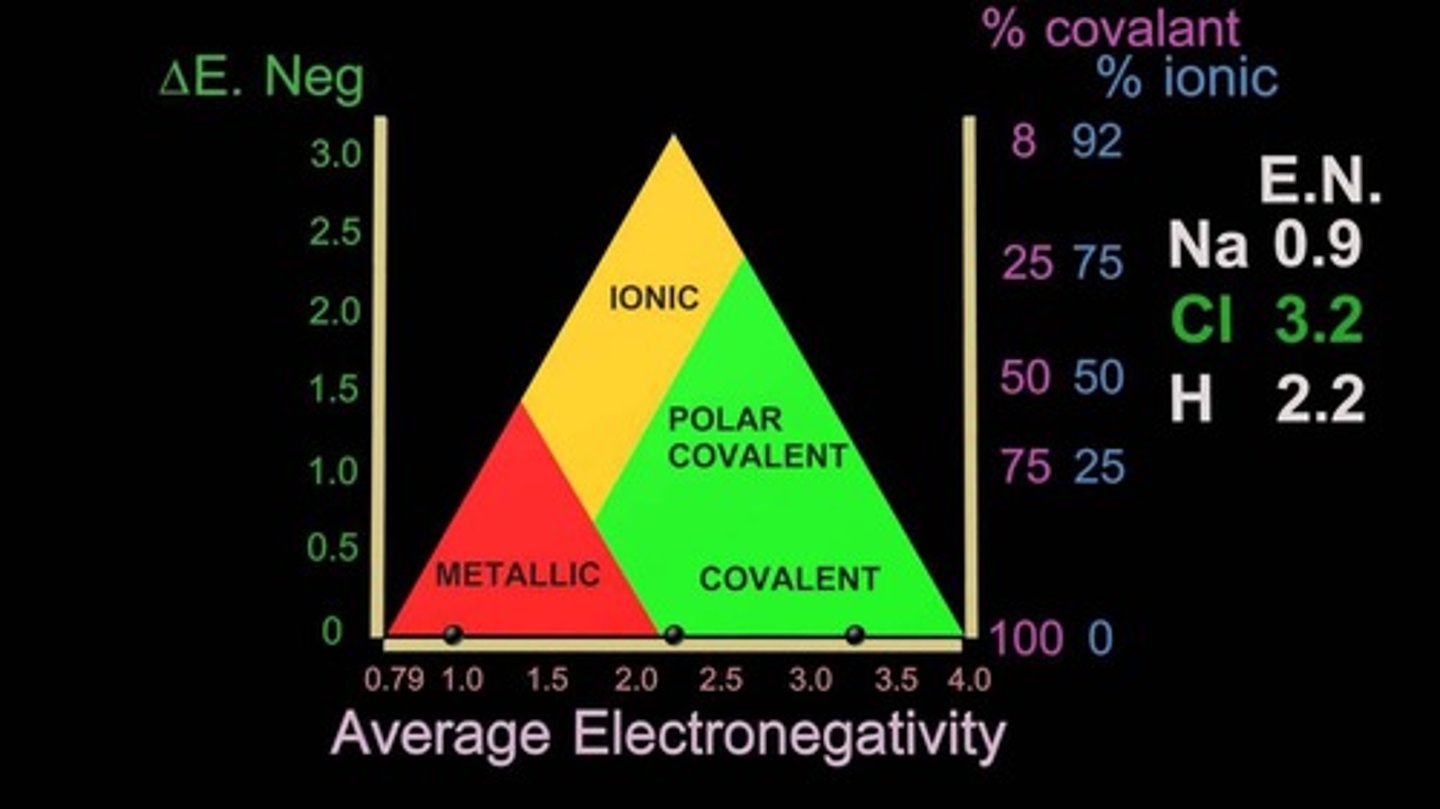
NPC, PC, vs I EN values
The difference btwn the two values (Y) helps find the bond type.
Non Polar: 0.0 - 0.4
Polar: 0.41 - 1.7
Ionic: 1.7+
The larger the difference, the more ionic it is.
Properties of Covalent Compounds
1. Lower melting point
- Typically. The MP value itself depends on the strength of the attraction of the two elements.
2. Low/Poor conductivity
- Typically. Some Polar Cov will produce ions to show conductivity.
3. All states of matter
- depends on the strength of the attraction
4. Poor compression strength but good tensile strength
5. "Like dissolves like"
- a compound's solubility in a specific context depends on its bond type. In any case, the more similar the polarity is to the "dissolver" the more likely it is to dissolve
- In water, the more polar (larger diff) it is, the more it will dissolve
Also: if it is flammable (becomes a gas quickly) it is Cov. Examples for Non Polar include Hexane and Lighter Fluid
Properties of Metallic Bonds/Metals
1. Ductile (able to be drawn into wire form due to the deloc. val e-)
2. Malleable (formable due to the deloc. val e- in the lattice
3. High Luster
4. Solids (except for Hg)
5. Good conductors
- Delocalized val e- + metal cations create a charge separation, allowing for movement of an electric current throughout the lattice
6. High melting point
Roving Sea of Electrons
In a metallic bond, electrons are free to roam which causes a charge separation leading to a metallic bond